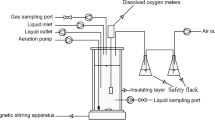
Abstract
The effects of temperature on nitrous oxide (N2O) accumulation during denitrification and denitritation were investigated. Batch experiments were performed to measure N2O accumulation at 25 and 35 °C. More N2O accumulation was observed during denitritation at the higher temperature as compared with full denitrification and low temperature tests. The highest nitrite concentration tested in this study (25 mg/L NO2 −N and pH 8.0) did not show inhibitory effect on N2O reduction. It was found that the major cause of more N2O accumulation during denitrification at higher temperature was due to higher N2O production rate and lower N2O solubility. Specific nitrate, nitrite, and N2O reduction rates increased 62, 61, and 41 %, respectively, when temperature rose from 25 to 35 °C. The decrease of N2O solubility in mixed liquor at 35 °C (when compared to 25 °C) resulted in faster diffusing rate of N2O from liquid to gas phase. It was also more difficult for gas phase N2O to be re-dissolved. The diffused N2O was then accumulated in the headspace, which was not available for denitrification by denitrifiers. The results of this study suggest higher temperature may worsen N2O emission from wastewater treatment plants (WWTPs).






Similar content being viewed by others
References
Ahn JH, Kim S, Park H, Rahm B, Pagilla K, Chandran K (2010) N2O emissions from activated sludge processes, 2008-2009: results of a national monitoring survey in the United States. Environ Sci Technol 44(12):4505–4511. doi:10.1021/es903845y
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous acid. J Water Pollut Control Fed 48(5):835–852
APHA (2012) Standard methods for examination of water and wastewater. American Public Health Association, Washington DC
Baytshtok V, Lu HJ, Park H, Kim S, Yu R, Chandran K (2009) Impact of varying electron donors on the molecular microbial ecology and biokinetics of methylotrophic denitrifying bacteria. Biotechnol Bioeng 102(6):1527–1536. doi:10.1002/bit.22213
Clarke JKA (1964) Kinetics of absorption of cardon dioxide in monoethanolamine solutions at short contact times. Ind Eng Chem Fundam 3(3):239–245. doi:10.1021/i160011a012
Dawson RN, Murphy KL (1972) The temperature dependency of biological denitrification. Water Res 6(1):71–83. doi:10.1016/0043-1354(72)90174-1
Gejlsbjerg B, Frette L, Westermann P (1998) Dynamics of N2O production from activated sludge. Water Res 32(7):2113–2121. doi:10.1016/s0043-1354(97)00456-9
Haimour N, Sandall OC (1984) Absorption of carbon dioxide into aqueous methyldiethanolamine. Chem Eng Sci 39(12):1791–1796. doi:10.1016/0009-2509(84)80115-3
Haynes WM (2013) CRC handbook of chemistry and physics, 94th edn. Taylor & Francis, Florida
Hiatt WC, Grady CPL (2008) Application of the activated sludge model for nitrogen to elevated nitrogen conditions. Water Environ Res 80(11):2134–2144. doi:10.2175/106143008x304767
Hu Z, Zhang J, Li S, Xie H (2013) Impact of carbon source on nitrous oxide emission from anoxic/oxic biological nitrogen removal process and identification of its emission sources. Environ Sci Pollut Res 20(2):1059–1069. doi:10.1007/s11356-012-1018-6
IPCC (2001) Climate change 2001: the scientific basis. Cambridge Univ. Press, Cambridge
Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MSM, van Loosdrecht MCM (2009) Nitrous oxide emission during wastewater treatment. Water Res 43(17):4093–4103. doi:10.1016/j.watres.2009.03.001
Lewandoswki Z (1982) Temperature dependency of biological denitrification with organic materials addition. Water Res 16(1):19–22. doi:10.1016/0043-1354(82)90048-3
Li C, Zhang J, Liang S, Ngo H, Guo W, Zhang Y, Zou Y (2013) Nitrous oxide generation in denitrifying phosphorus removal process: main causes and control measures. Environ Sci Pollut Res 20(8):5353–5360. doi:10.1007/s11356-013-1530-3
Lu HJ, Chandran K (2010) Factors promoting emissions of nitrous oxide and nitric oxide from denitrifying sequencing batch reactors operated with methanol and ethanol as electron donors. Biotechnol Bioeng 106(3):390–398. doi:10.1002/bit.22704
Metcalf and Eddy, Tchobanoglous G, Burton FL, Stensel HD (2013) Wastewater engineering: treatment and resource recovery. McGraw-Hill Education, New York, USA
Pan Y, Ye L, Ni B-J, Yuan Z (2012) Effect of pH on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Water Res 46(15):4832–4840. doi:10.1016/j.watres.2012.06.003
Pan Y, Ni B-J, Bond PL, Ye L, Yuan Z (2013a) Electron competition among nitrogen oxides reduction during methanol-utilizing denitrification in wastewater treatment. Water Res 47(10):3273–3281. doi:10.1016/j.watres.2013.02.054
Pan Y, Ye L, Yuan Z (2013b) Effect of H2S on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Environ Sci Technol 47(15):8408–8415. doi:10.1021/es401632r
Ribera-Guardia A, Kassotaki E, Gutierrez O, Pijuan M (2014) Effect of carbon source and competition for electrons on nitrous oxide reduction in a mixed denitrifying microbial community. Process Biochem 49(12):2228–2234. doi:10.1016/j.procbio.2014.09.020
Richardson D, Felgate H, Watmough N, Thomson A, Baggs E (2009) Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle—could enzymic regulation hold the key? Trends Biotechnol 27(7):388–397. doi:10.1016/j.tibtech.2009.03.009
Schreiber F, Loeffler B, Polerecky L, Kuypers MMM, de Beer D (2009) Mechanisms of transient nitric oxide and nitrous oxide production in a complex biofilm. ISME J 3(11):1301–1313. doi:10.1038/ismej.2009.55
Versteeg GF, Van Swaalj W (1988) Solubility and diffusivity of acid gases (carbon dioxide, nitrous oxide) in aqueous alkanolamine solutions. J Chem Eng Data 33(1):29–34. doi:10.1021/je00051a011
Weiss RF, Price BA (1980) Nitrous oxide solubility in water and seawater. Mar Chem 8(4):347–359. doi:10.1016/0304-4203(80)90024-9
Yaghi B, Houache O (2008) Solubility of nitrous oxide in amine aqueous solutions. J Eng Comput Arch 2(1):1-14
Zeng RJ, Yuan ZG, Keller J (2003) Enrichment of denitrifying glycogen-accumulating organisms in anaerobic/anoxic activated sludge system. Biotechnol Bioeng 81(4):397–404. doi:10.1002/bit.10484
Zhou Y, Pijuan M, Zeng RJ, Yuan Z (2008) Free nitrous acid inhibition on nitrous oxide reduction by a denitrifying-enhanced biological phosphorus removal sludge. Environ Sci Technol 42(22):8260–8265. doi:10.1021/es800650j
Zhou Y, Ganda L, Lim M, Yuan ZG, Kjelleberg S, Ng WJ (2010) Free nitrous acid (FNA) inhibition on denitrifying poly-phosphate accumulating organisms (DPAOs). Appl Microbiol Biotechnol 88(1):359–369. doi:10.1007/s00253-010-2780-3
Zhou Y, Oehmen A, Lim M, Vadivelu V, Ng WJ (2011) The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res 45(15):4672–4682. doi:10.1016/j.watres.2011.06.025
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61(4):533–616
Acknowledgments
This project is supported by funding from Ministry of Environment and Water Resources, Singapore (MEWR), Environment and Water Industry Programme Office (EWI) through project “Energy +: A novel integrated concept for retrofitting and optimizing existing wastewater treatment plants into energy self-sufficiency,” project reference number 2P 10004/95.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Poh, L.S., Jiang, X., Zhang, Z. et al. N2O accumulation from denitrification under different temperatures. Appl Microbiol Biotechnol 99, 9215–9226 (2015). https://doi.org/10.1007/s00253-015-6742-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6742-7