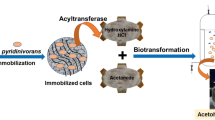
Abstract
A novel (+) γ-lactamase gene (rutB) was cloned from Escherichia coli JM109 and expressed in E. coli BL21 (DE3), and the recombinant protein was characterized. The optimal conditions for the enzyme were pH 7.0 and temperature 30 °C, which indicated that it was a mesophilic protein. The free purified enzyme was deactivated when incubated at 50 °C for 30 min. However, the k cat value of RutB at its optimal temperature was about 2.5 times that of the archaeal enzyme from Sulfolobus sofataricus at its optimal temperature (85 °C). After immobilization on macroporous resin using glutaraldehyde cross-linkage, the thermostability of the crude enzyme was greatly enhanced and the deactivating temperature was raised to 70 °C. After immobilization, the minimal substrate inhibition concentration for RutB also improved from 0.75 to 1.5 M. The optimal concentrations of immobilized enzyme and substrate were determined to be 250 mg/ml and 1.5 M, when the initial reaction velocity was the response variable in batch transformations. This immobilization of RutB on macroporous resins provides another feasible approach for the preparation of optically active Vince lactam. As a member of the isochorismatase superfamily, RutB was demonstrated to be another typical γ-lactamase that showed catalytic promiscuity.





Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. doi:10.1006/jmbi.1990.9999
Ben Mabrouk S, Ayadi DZ, Ben Hlima H, Bejar S (2013) Thermostability improvement of maltogenic amylase MAUS149 by error prone PCR. J Biotechnol 168(4):601–606. doi:10.1016/j.jbiotec.2013.08.026
Caruthers J, Zucker F, Worthey E, Myler PJ, Buckner F, Van Voorhuis W, Mehlin C, Boni E, Feist T, Luft J, Gulde S, Lauricella A, Kaluzhniy O, Anderson L, Le Trong I, Holmes MA, Earnest T, Soltis M, Hodgson KO, Hol WG, Merritt EA (2005) Crystal structures and proposed structural/functional classification of three protozoan proteins from the isochorismatase superfamily. Protein Sci 14(11):2887–2894. doi:10.1110/ps.051783005
Cilia E, Fabbri A, Uriani M, Scialdone GG, Ammendola S (2005) The signature amidase from Sulfolobus solfataricus belongs to the CX3C subgroup of enzymes cleaving both amides and nitriles. Ser195 and Cys145 are predicted to be the active site nucleophiles. FEBS J 272(18):4716–4724. doi:10.1111/j.1742-4658.2005.04887.x
Deng Z, Yang H, Shin HD, Li J, Liu L (2014) Structure-based rational design and introduction of arginines on the surface of an alkaline alpha-amylase from Alkalimonas amylolytica for improved thermostability. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-5790-8
Ellis RJ (2001) Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci 26(10):597–604
Ghollasi M, Ghanbari-Safari M, Khajeh K (2013) Improvement of thermal stability of a mutagenised alpha-amylase by manipulation of the calcium-binding site. Enzym Microb Technol 53(6–7):406–413. doi:10.1016/j.enzmictec.2013.09.001
Gonsalvez IS, Isupov MN, Littlechild JA (2001) Crystallization and preliminary X-ray analysis of a gamma-lactamase. Acta Crystallogr D Biol Crystallogr 57(Pt 2):284–286
Goral AM, Tkaczuk KL, Chruszcz M, Kagan O, Savchenko A, Minor W (2012) Crystal structure of a putative isochorismatase hydrolase from Oleispira antarctica. J Struct Funct Genom 13(1):27–36. doi:10.1007/s10969-012-9127-5
Gubareva LV, Webster RG, Hayden FG (2001) Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother 45(12):3403–3408. doi:10.1128/AAC. 45.12.3403-3408.2001
Hogrefe HH, Cline J, Youngblood GL, Allen RM (2002) Creating randomized amino acid libraries with the QuikChange multi site-directed mutagenesis kit. Biotechniques 33(5):1158–1160, 1162, 1164–5
Jagt JC, Van Leusen AM (1974) Diels-Alder cycloadditions of sulfonyl cyanides with cyclopentadiene. Synthesis of 2-azabicyclo[2.2.1]hepta-2,5-dienes. J Org Chem 39(4):564–566. doi:10.1021/jo00918a033
Khersonsky O, Tawfik DS (2010) Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu Rev Biochem 79:471–505. doi:10.1146/annurev-biochem-030409-143718
King CH, Meckler H, Herr RJ, Trova MP, Glick SD, Maisonneuve IM (2000) Synthesis of enantiomerically pure (+)- and (−)-18-methoxycoronaridine hydrochloride and their preliminary assessment as anti-addictive agents. Bioorg Med Chem Lett 10(5):473–476
Klibanov AM (1979) Enzyme stabilization by immobilization. Anal Biochem 93(1):1–25
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Li HQ, Su L, Yang L, Wang JJ, Zheng GJ (2006) Study on the screening of lactamase and its fermentation conditions. Wei Sheng Wu Xue Bao 46(4):571–575
Li X, Huang S, Xu L, Yan Y (2013) Improving activity and enantioselectivity of lipase via immobilization on macroporous resin for resolution of racemic 1- phenylethanol in non-aqueous medium. BMC Biotechnol 13:92. doi:10.1186/1472-6750-13-92
Line K, Isupov MN, Littlechild JA (2004) The crystal structure of a (−) gamma-lactamase from an Aureobacterium species reveals a tetrahedral intermediate in the active site. J Mol Biol 338(3):519–532. doi:10.1016/j.jmb.2004.03.001S0022283604002633
Lopez-Gallego F, Betancor L, Mateo C, Hidalgo A, Alonso-Morales N, Dellamora-Ortiz G, Guisan JM, Fernandez-Lafuente R (2005) Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J Biotechnol 119(1):70–75. doi:10.1016/j.jbiotec.2005.05.021
Martinek K, Klibanov AM, Goldmacher VS, Berezin IV (1977) The principles of enzyme stabilization. I. Increase in thermostability of enzymes covalently bound to a complementary surface of a polymer support in a multipoint fashion. Biochim Biophys Acta 485(1):1–12
Mateo C, Monti R, Pessela BC, Fuentes M, Torres R, Guisan JM, Fernandez-Lafuente R (2004) Immobilization of lactase from Kluyveromyces lactis greatly reduces the inhibition promoted by glucose. Full hydrolysis of lactose in milk. Biotechnol Prog 20(4):1259–1262. doi:10.1021/bp049957m
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym Microb Technol 40(6):1451–1463. doi:10.1016/j.enzmictec.2007.01.018
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37(5):790–796, 798–802
Minton AP (2001) The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem 276(14):10577–10580. doi:10.1074/jbc.R100005200
Mozhaev VV, Melik-nubarov NS, Sergeeva MV, Šikšnis V, Martinek K (1990) Strategy for stabilizing enzymes part one: increasing stability of enzymes via their multi-point interaction with a support. Biocatalysis Biotransformation 3(3):179–187. doi:10.3109/10242429008992060
Nastopoulos V, Vallone B, Politi L, Scotto D’Abusco A, Scandurra R, Tsernoglou D (2001) Crystallization and X-ray diffraction measurements of a thermophilic archaeal recombinant amidase from Sulfolobus solfataricus MT4. Acta Crystallogr D Biol Crystallogr 57(Pt 7):1036–1037
Parsons JF, Calabrese K, Eisenstein E, Ladner JE (2003) Structure and mechanism of Pseudomonas aeruginosa PhzD, an isochorismatase from the phenazine biosynthetic pathway. Biochemistry 42(19):5684–5693. doi:10.1021/bi027385d
Pessela BCC, Mateo C, Fuentes M, Vian A, Garcia JL, Carrascosa AV, Guisan JM, Fernandez-Lafuente R (2003) The immobilization of a thermophilic beta-galactosidase on Sepabeads supports decreases product inhibition—complete hydrolysis of lactose in dairy products. Enzym Microb Technol 33(2–3):199–205. doi:10.1016/S0141-0229(03)00120-0
Qin X, Wang J, Zheng G (2010) Enantioselective resolution of gamma-lactam by a whole cell of Microbacterium hydrocarbonoxydans (L29-9) immobilized in polymer of PVA-alginate-boric acid. Appl Biochem Biotechnol 162(8):2345–2354. doi:10.1007/s12010-010-9007-z
Radestock S, Gohlke H (2011) Protein rigidity and thermophilic adaptation. Proteins 79(4):1089–1108. doi:10.1002/prot.22946
Reed MC, Lieb A, Nijhout HF (2010) The biological significance of substrate inhibition: a mechanism with diverse functions. Bioessays 32(5):422–429. doi:10.1002/bies.200900167
Silva IR, Jers C, Otten H, Nyffenegger C, Larsen DM, Derkx PM, Meyer AS, Mikkelsen JD, Larsen S (2014) Design of thermostable rhamnogalacturonan lyase mutants from Bacillus licheniformis by combination of targeted single point mutations. Appl Microbiol Biotechnol 98(10):4521–4531. doi:10.1007/s00253-013-5483-8
Singh R, Vince R (2012) 2-Azabicyclo[2.2.1]hept-5-en-3-one: chemical profile of a versatile synthetic building block and its impact on the development of therapeutics. Chem Rev 112(8):4642–4686. doi:10.1021/cr2004822
Suzuki T, Usui T, Oka M, Kataoka T (1998) Synthesis and muscarinic activity of a series of quinolines and naphthalenes with a 1-azabicyclo[3.3.0]octane moiety. Chem Pharm Bull (Tokyo) 46(8):1265–1273
Tanaka K, Kato M, Toda F (2001) Optical resolution of 2-azabicyclo[2.2.1]hept-5-en-3-one by inclusion complexation with brucine. Heterocycles 54(1):405–410
Taylor JD (2010) COPD and the response of the lung to tobacco smoke exposure. Pulm Pharmacol Ther 23(5):376–383. doi:10.1016/j.pupt.2010.04.003
Taylor SJC, Mccague R, Wisdom R, Lee C, Dickson K, Ruecroft G, Obrien F, Littlechild J, Bevan J, Roberts SM, Evans CT (1993) Development of the biocatalytic resolution of 2-azabicyclo [2.2.1]hept-5-En-3-one as an entry to single-enantiomer carbocyclic nucleosides. Tetrahedron-Asymmetry 4(6):1117–1128. doi:10.1016/S0957-4166(00)80218-9
Toogood HS, Brown RC, Line K, Keene PA, Taylor SJC, McCague R, Littlechild JA (2004) The use of a thermostable signature amidase in the resolution of the bicyclic synthon (rac)-gamma-lactam. Tetrahedron 60(3):711–716. doi:10.1016/j.tet.2003.11.064
Torres LL, Schliessmann A, Schmidt M, Silva-Martin N, Hermoso JA, Berenguer J, Bornscheuer UT, Hidalgo A (2012) Promiscuous enantioselective (−)-[gamma]-lactamase activity in the Pseudomonas fluorescens esterase I. Org Biomol Chem 10(17):3388–3392. doi:10.1039/c2ob06887g
Wang JJ, Zhang X, Min C, Wu S, Zheng GJ (2011) Single-step purification and immobilization of gamma-lactamase and on-column transformation of 2-azabicyclo [2.2.1] hept-5-en-3-one. Process Biochem 46(1):81–87. doi:10.1016/j.procbio.2010.07.018
Wang J, Zhu J, Min C, Wu S (2014a) CBD binding domain fused gamma-lactamase from Sulfolobus solfataricus is an efficient catalyst for (−) gamma-lactam production. BMC Biotechnol 14(1):40
Wang J, Zhu Y, Zhao G, Zhu J, Wu S (2014b) Characterization of a recombinant (+)-gamma-lactamase from Microbacterium hydrocarbonoxydans which provides evidence that two enantiocomplementary gamma-lactamases are in the strain. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-6114-8
Wang K, Luo H, Tian J, Turunen O, Huang H, Shi P, Hua H, Wang C, Wang S, Yao B (2014c) Thermostability improvement of a streptomyces xylanase by introducing proline and glutamic acid residues. Appl Environ Microbiol 80(7):2158–2165. doi:10.1128/AEM. 03458-13
Warmerdam A, Boom R, Janssen A (2013) β-galactosidase stability at high substrate concentrations. Springer Plus 2(1):1–8. doi:10.1186/2193-1801-2-402
Wen YD, Remmel RP, Pham PT, Vince R, Zimmerman CL (1995) Comparative brain exposure to (−)-carbovir after (−)-carbovir or (−)-6-aminocarbovir intravenous infusion in rats. Pharm Res 12(6):911–915
Wisniewski T, Bayne E, Flanagan J, Shao Q, Wnek R, Matheravidathu S, Fischer P, Forrest MJ, Peterson L, Song X, Yang L, Demartino JA, Struthers M (2010) Assessment of chemokine receptor function on monocytes in whole blood: in vitro and ex vivo evaluations of a CCR2 antagonist. J Immunol Methods 352(1–2):101–110. doi:10.1016/j.jim.2009.10.010
Xiu GH, Jiang L, Li P (2001) Mass-transfer limitations for immobilized enzyme-catalyzed kinetic resolution of racemate in a fixed-bed reactor. Biotechnol Bioeng 74(1):29–39
Acknowledgments
This work was supported by National Natural Science Foundation of China no. 31070718.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 76 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Zhu, J. & Wu, S. Immobilization on macroporous resin makes E. coli RutB a robust catalyst for production of (−) Vince lactam. Appl Microbiol Biotechnol 99, 4691–4700 (2015). https://doi.org/10.1007/s00253-014-6247-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6247-9