Abstract
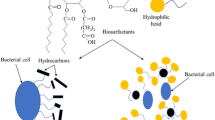
Actinomycetales are known to produce various secondary metabolites including products with surface-active and emulsifying properties known as biosurfactants. In this study, the nonpathogenic actinomycetes Tsukamurella spumae and Tsukamurella pseudospumae are described as producers of extracellular trehalose lipid biosurfactants when grown on sunflower oil or its main component glyceryltrioleate. Crude extracts of the trehalose lipids were purified using silica gel chromatography. The structure of the two trehalose lipid components (TL A and TL B) was elucidated using a combination of matrix-assisted laser desorption/ionization time-of-flight/time-of-flight/tandem mass spectroscopy (MALDI-ToF-ToF/MS/MS) and multidimensional NMR experiments. The biosurfactants were identified as 1-α-glucopyranosyl-1-α-glucopyranosid carrying two acyl chains varying of C4 to C6 and C16 to C18 at the 2′ and 3′ carbon atom of one sugar unit. The trehalose lipids produced demonstrate surface-active behavior and emulsifying capacity. Classified as risk group 1 organisms, T. spumae and T. pseudospumae hold potential for the production of environmentally friendly surfactants.








Similar content being viewed by others
References
Asselineau C, Asselineau J (1978) Trehalose-containing glycolipids. Prog Chem Fats Other Lipids 16:59–99
Azuma M, Suzutani T, Sazaki K, Yoshida I, Sakuma T, Yoshida T (1987) Role of interferon in the augmented resistance of trehalose-6, 6′-dimycolate-treated mice to influenza virus infection. J Gen Virol 68:835–843
Baltz RH (2008) Renaissance in antibacterial discovery from actinomycetes. Curr Opin Pharmacol 8(5):557–563
Berdy J (2005) Bioactive microbial metabolites. J Antibiot 58(1):1–26
Bicca FC, Fleck LC, Ayub MAZ (1999) Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Rev Microbiol 30(3):231–236
Cameotra SS, Makkar RS (2004) Recent applications of biosurfactants as biological and immunological molecules. Curr Opin Microbiol 7(3):262–266. doi:10.1016/j.mib.2004.04.006
Choi KS, Kim SH, Lee TH (1999) Purification and characterization of biosurfactant from Tsukamurella sp. 26A. J Microbiol Biotechnol 9(1):32–38
Christofi N, Ivshina I (2002) Microbial surfactants and their use in field studies of soil remediation. J Appl Microbiol 93(6):915–929
Daniel H-J, Reuss M, Syldatk C (1998) Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in a two stage fed batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotechnol Lett 20(12):1153–1156. doi:10.1023/a:1005332605003
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61(1):47–64
DSMZ (webpage) Leibnitz Institute—German collection of microorganisms and cell cultures, catalogue of microorganisms. http://www.dsmz.de/catalogues/details/culture/DSM-44113.html Accessed: 23.05.2014
Du Noüy PL (1919) A new apparatus for measuring surface tension. J Gen Physiol 1(5):521
Embley T, Stackebrandt E (1994) The molecular phylogeny and systematics of the actinomycetes. Annu Rev Microbiol 48(1):257–289
Franzetti A, Gandolfi I, Bestetti G, Smyth TJ, Banat IM (2010) Production and applications of trehalose lipid biosurfactants. Eur J Lipid Sci Technol 112(6):617–627
Gudiña EJ, Rangarajan V, Sen R, Rodrigues LR (2013) Potential therapeutic applications of biosurfactants. Trends Pharmacol Sci 34(12):667–675
Henkel M, Müller MM, Kügler JH, Lovaglio RB, Contiero J, Syldatk C, Hausmann R (2012) Rhamnolipids as biosurfactants from renewable resources: concepts for next-generation rhamnolipid production. Process Biochem 47(8):1207–1219
Iwahori K, Tokutomi T, Miyata N, Fujita M (2001) Formation of stable foam by the cells and culture supernatant of Gordonia (Nocardia) amarae. J Biosci Bioeng 92(1):77–79
Jackisch-Matsuura AB, Santos LS, Eberlin MN, AiFd F, Matsuura T, Grossman MJ, Durrant LR (2014) Production and characterization of surface-active compounds from Gordonia amicalis. Braz Arch Biol Technol 57(1):138–144
Khopade A, Ren B, Liu X-Y, Mahadik K, Zhang L, Kokare C (2011) Production and characterization of biosurfactant from marine Streptomyces species B3. J Colloid Interface Sci 367(1):311–318
Kim SH, Lim EJ, Lee SO, Lee JD, Lee TH (2000) Purification and characterization of biosurfactants from Nocardia sp. L-417. Biotechnol Appl Biochem 31(3):249–253
Kiran GS, Thomas TA, Selvin J (2010) Production of a new glycolipid biosurfactant from marine Nocardiopsis lucentensis MSA04 in solid-state cultivation. Colloids Surf B 78(1):8–16. doi:10.1016/j.colsurfb.2010.01.028
Kuyukina MS, Ivshina IB (2010) Application of Rhodococcus in bioremediation of contaminated environments. In: Alvarez HM (ed) Biology of Rhodococcus. Springer, Berlin, pp 231–262
Lang S, Philp JC (1998) Surface-active lipids in rhodococci. Anton Leeuw 74(1–3):59–70
Marat K Spinworks 3.1.8. University of Manitoba ftp://davinci.chem.umanitoba.ca/pub/marat/SpinWorks/
Marchant R, Banat IM (2012) Microbial biosurfactants: challenges and opportunities for future exploitation. Trends Biotechnol 30(11):558–565. doi:10.1016/j.tibtech.2012.07.003
Morikawa M, Daido H, Takao T, Murata S, Shimonishi Y, Imanaka T (1993) A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J Bacteriol 175(20):6459–6466
Müller MM, Kügler JH, Henkel M, Gerlitzki M, Hörmann B, Pöhnlein M, Syldatk C, Hausmann R (2012) Rhamnolipids—next generation surfactants? J Biotechnol 162(4):366–380
Nam S-W, Chun J, Kim S, Kim W, Zakrzewska-Czerwinska J, Goodfellow M (2003) Tsukamurella spumae sp. nov., a novel actinomycete associated with foaming in activated sludge plants. Syst Appl Microbiol 26(3):367–375. doi:10.1078/072320203322497392
Nam S-W, Kim W, Chun J, Goodfellow M (2004) Tsukamurella pseudospumae sp. nov., a novel actinomycete isolated from activated sludge foam. Int J Syst Evol Microbiol 54(4):1209–1212
Niescher S, Wray V, Lang S, Kaschabek SR, Schlömann M (2006) Identification and structural characterisation of novel trehalose dinocardiomycolates from n-alkane-grown Rhodococcus opacus 1CP. Appl Microbiol Biotechnol 70(5):605–611
Philp J, Kuyukina M, Ivshina I, Dunbar S, Christofi N, Lang S, Wray V (2002) Alkanotrophic Rhodococcus ruber as a biosurfactant producer. Appl Microbiol Biotechnol 59(2–3):318–324
Powalla M, Lang S, Wray V (1989) Penta- and disaccharide lipid formation by Nocardia corynebacteroides grown on n-alkanes. Appl Microbiol Biotechnol 31(5–6):473–479
Richter M, Willey JM, Süßmuth R, Jung G, Fiedler H-P (1998) Streptofactin, a novel biosurfactant with aerial mycelium inducing activity from Streptomyces tendae Tü 901/8c. FEMS Microbiol Lett 163(2):165–171. doi:10.1111/j.1574-6968.1998.tb13041.x
Ristau E, Wagner F (1983) Formation of novel anionic trehalosetetraesters from Rhodococcus erythropolis under growth limiting conditions. Biotechnol Lett 5(2):95–100
Shao Z (2011) Trehalolipids. In: Soberón-Chávez G (ed) Biosurfactants—from genes to applications. Springer Berlin Heidelberg, pp 121-143
Sudo T, Zhao X, Wakamatsu Y, Shibahara M, Nomura N, Nakahara T, Suzuki A, Kobayashi Y, Jin C, Murata T (2000) Induction of the differentiation of human HL-60 promyelocytic leukemia cell line by succinoyl trehalose lipids. Cytotechnology 33(1–3):259–264
Tokumoto Y, Nomura N, Uchiyama H, Imura T, Morita T, Fukuoka T, Kitamoto D (2008) Structural characterization and surface-active properties of a succinoyl trehalose lipid produced by Rhodococcus sp. SD-74. J Oleo Sci 58(2):97–102
Vollbrecht E, Heckmann R, Wray V, Nimtz M, Lang S (1998) Production and structure elucidation of di- and oligosaccharide lipids (biosurfactants) from Tsukamurella sp. nov. Appl Microbiol Biotechnol 50(5):530–537
Watanabe R, Yoo YC, Hata K, Mitobe M, Koike Y, Nishizawa M, Garcia DM, Nobuchi Y, Imagawa H, Yamada H (1999) Inhibitory effect of trehalose dimycolate (TDM) and its stereoisometric derivatives, trehalose dicorynomycolates (TDCMs), with low toxicity on lung metastasis of tumour cells in mice. Vaccine 17(11):1484–1492
Zaragoza A, Aranda FJ, Espuny MJ, Teruel JA, Marqués A, Manresa A, Ortiz A (2009) Mechanism of membrane permeabilization by a bacterial trehalose lipid biosurfactant produced by Rhodococcus sp. Langmuir 25(14):7892–7898
Acknowledgments
The authors acknowledge the German Federal Ministry of Education and Research for funding this project (0315928B) as part of the ERA-IB BioSurf project (617 4003 0315928B).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 543 kb)
Rights and permissions
About this article
Cite this article
Kügler, J.H., Muhle-Goll, C., Kühl, B. et al. Trehalose lipid biosurfactants produced by the actinomycetes Tsukamurella spumae and T. pseudospumae . Appl Microbiol Biotechnol 98, 8905–8915 (2014). https://doi.org/10.1007/s00253-014-5972-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5972-4