Abstract
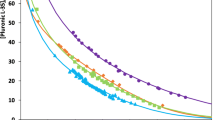
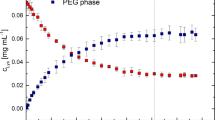
Lipopolysaccharide endotoxins (LPS) are the most common pyrogenic substances in recombinant peptides and proteins purified from Gram-negative bacteria, such as Escherichia coli. In this respect, aqueous two-phase micellar systems (ATPMS) have already proven to be a good strategy to purify recombinant proteins of pharmaceutical interest and remove high LPS concentrations. In this paper, we review our recent experimental work in protein partitioning in Triton X-114 ATPMS altogether with some new results and show that LPS–protein aggregation can influence both protein and LPS partitioning. Green fluorescent protein (GFPuv) was employed as a model protein. The ATPMS technology proved to be effective for high loads of LPS removal into the micelle-rich phase (%REMLPS > 98 %) while GFPuv partitioned preferentially to the micelle-poor phase (K GFPuv < 1.00) due to the excluded-volume interactions. However, theoretically predicted protein partition coefficient values were compared with experimentally obtained ones, and good agreement was found only in the absence of LPS. Dynamic light scattering measurements showed that protein–LPS interactions were taking place and influenced the partitioning process. We believe that this phenomenon should be considered in LPS removal employing any kind of aqueous two-phase system. Nonetheless, ATPMS can still be considered as an efficient strategy for high loads of LPS removal, but being aware that the excluded-volume partitioning theory available might overestimate partition coefficient values due to the presence of protein–LPS aggregation.






Similar content being viewed by others
References
Albertsson PÅ (1986) Partition of cell particles and macromolecules, 3rd edn. Wiley, New York, 346 p
Anspach FB (2001) Endotoxin removal by affinity sorbents. J Biochem Biophys Methods 49:661–681
Bokman SH, Ward WW (1981) Renaturation of Aequorea green fluorescent protein. Biochem Biophsy Res Commun 101:1372–1380
Brömme D, Nallaseth FS, Turk B (2004) Production and activation of recombinant papain-like cysteine proteases. Methods 32:199–206
Chen R (2012) Bacterial expression systems for recombinant protein production: E. coli and beyond. Biotechnol Adv 30:1102–1107
Fischer I, Morhardt C, Heissler S, Franzreb M (2012) Partitioning behavior of silica-coated nanoparticles in aqueous micellar two-phase systems: evidence for an adsorption-driven mechanism from QCM-D and ATR-FTIR measurements. Langmuir 28:15789–15796
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Hou KC, Zaniewski R (1990) Depyrigenation by endotoxin removal with positively charged depth filter cartridge. J Parenter Sci Technol 44:204–209
Kamei DT, Wang DIC, Blankschtein D (2002) Fundamental investigation of protein partitioning in two-phase aqueous mixed (nonionic/ionic) micellar systems. Langmuir 18:3047–3057
Liu CL, Nikas YJ, Blankschtein D (1996) Novel bioseparations using two-phase aqueous micellar systems. Biotechnol Bioeng 52:185–192
Lopes AM, Rangel-Yagui CO, Pessoa-Jr A (2008) Can affinity interactions influence the partitioning of glucose-6-phosphate dehydrogenase in two-phase aqueous micellar systems? Quím Nova 31:998–1003
Lopes AM, Magalhães PO, Mazzola PG, Rangel-Yagui CO, Carvalho JCM, Penna TCV, Pessoa-Jr A (2010) LPS removal from an E. coli fermentation broth using aqueous two-phase micellar system. Biotechnol Prog 26:1644–1653
Lopes AM, Magalhães PO, Mazzola PG, Rangel-Yagui CO, Carvalho JCM, Penna TCV, Pessoa-Jr A (2011) Green fluorescent protein extraction and LPS removal from E. coli fermentation medium using aqueous two-phase micellar system. Sep Purif Technol 81:339–346
Lopes AM, Romeu JS, Perera GM, Paez R, Morales RP, Pessoa-JR A, Cardenas LZ (2012) Adsorption of endotoxins on IDA-Ca2+ by ion metal affinity chromatography. Chin J Chromatogr 30:1194–1202
Lue L, Blankschtein D (1996) A liquid-state theory approach to modeling solute partitioning in phase-separated solutions. Ind Eng Chem Res 35:3032–3043
Magalhães PO, Lopes AM, Mazzola PG, Rangel-Yagui CO, Penna TCV, Pessoa-Jr A (2007) Methods of endotoxin removal from biological preparations: a review. J Pharm Pharm Sci 10:271–287
Mazzola PG, Lam H, Kavoosi M, Haynes CA, Pessoa-Jr A, Penna TCV, Haynes CA, Wang DIC, Blankschtein D (2006) Affinity-tagged green fluorescent protein (GFPuv) extraction from a clarified E. coli cell lysate using a two-phase aqueous micellar system. Biotechnol Bioeng 93:998–1004
Mazzola PG, Lopes AM, Hasmann FA, Jozala AF, Penna TCV, Magalhaes PO, Rangel-Yagui CO, Pessoa-Jr A (2008) Liquid–liquid extraction of biomolecules: an overview and update of the main techniques. J Chem Technol Biot 83:143–157
Nikas YJ, Liu CL, Srivastava T, Abbott NL, Blankschtein D (1992) Protein partitioning in two-phase aqueous nonionic micellar solutions. Macromolecules 25:4794–4806
Primrose SB, Twyman R (2006) Principles of gene manipulation and genomics. Wiley-Blackwell, New York, 672 p
Puvvada S, Blankschtein D (1990) Molecular-thermodynamic approach to predict micellization, phase behavior, and phase separation of micellar solutions. I. Application to nonionic surfactants approach. J Chem Phys 92:3710–3724
Rangel-Yagui CO, Lam H, Kamei DT, Wang D, Pessoa-Jr A, Blankschtein D (2003) Glucose-6-phosphate dehydrogenase partitioning in two-phase aqueous mixed (nonioc/cationic) micellar systems. Biotechnol Bioeng 82:445–456
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 72:211–222
US Pharmacopoeia-USP-26 (2002). US Pharmacopeial Convention, Rockville, 2569 p
US Pharmacopeia and National Formulary-USP-24-NF-19 (2000) Bacterial endotoxins. Test. Supplement 85:2875–2879
Westphal O, Jann K (1965) Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem 5:83–91
Yuan A, Pardy RL, Chia CP (1999) Nonspecific interactions alter lipopolysaccharide patterns and protein mobility on sodium dodecyl sulfate polyacrylamide gels. Electrophoresis 20:1946–1949
Zdziennicka A, Szymczyk K, Krawczyk J, Jańczuk B (2012) Critical micelle concentration of some surfactants and thermodynamic parameters of their micellization. Fluid Phase Equilib 322–323:126–134
Zimmer M (2002) Green fluorescent protein (GFP): applications, structure, and related photophysical behavior. Chem Rev 102:759–781
Acknowledgments
This research was supported by grants from the Coordination for Higher Level Graduate Improvements (CAPES/Brazil), National Council for Scientific and Technological Development (CNPq/Brazil), and State of São Paulo Research Foundation (FAPESP/Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopes, A.M., Santos-Ebinuma, V.d.C., Novaes, L.C.d.L. et al. LPS–protein aggregation influences protein partitioning in aqueous two-phase micellar systems. Appl Microbiol Biotechnol 97, 6201–6209 (2013). https://doi.org/10.1007/s00253-013-4922-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4922-x