Abstract
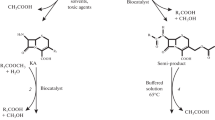
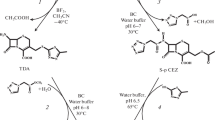
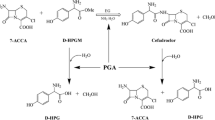
Cephalosporins currently constitute the most widely prescribed class of antibiotics and are used to treat diseases caused by both Gram-positive and Gram-negative bacteria. Cephalosporins contain a 7-aminocephalosporanic acid (7-ACA) nucleus which is derived from cephalosporin C (CephC). The 7-ACA nucleus is not sufficiently potent for clinical use; however, a series of highly effective antibiotic agents could be produced by modifying the side chains linked to the 7-ACA nucleus. The industrial production of higher-generation semi-synthetic cephalosporins starts from 7-ACA, which is obtained by deacylation of the naturally occurring antibiotic CephC. CephC can be converted to 7-ACA either chemically or enzymatically using d-amino acid oxidase and glutaryl-7-aminocephalosporanic acid acylase. Both these methods show limitation, including the production of toxic waste products (chemical process) and the expense (the enzymatic one). In order to circumvent these problems, attempts have been undertaken to design a single-step means of enzymatically converting CephC to 7-ACA in the course of the past 10 years. The most suitable approach is represented by engineering the activity of a known glutaryl-7-aminocephalosporanic acid acylase such that it will bind and deacylate CephC more preferentially over glutaryl-7-aminocephalosporanic acid. Here, we describe the state of the art in the production of an effective and specific CephC acylase.






Similar content being viewed by others
References
Anandan A, Vallet C, Coyle T, Moustafa IM, Vrielink A (2010) Crystallization and preliminary diffraction analysis of an engineered cephalosporin acylase. Acta Crystallogr Sect F Struct Biol Cryst Commun 66:808–810
Aramori I, Fukagawa M, Tsumura M, Iwami M, Yogota Y, Kojo H, Kohsaka M, Ueda Y, Imanaka H (1991a) Isolation of soil strains producing new cephalosporin acylases. J Ferment Bioeng 72:227–231
Aramori I, Fukagawa M, Tsumura M, Iwami M, Isogai T, Ono H, Ishitani Y, Kojo H, Kohsaka M, Ueda Y, Imanaka H (1991b) Cloning and nucleotide sequencing of new glutaryl 7-ACA and cephalosporin-C acylase genes from Pseudomonas strains. J Ferment Bioeng 72:232–243
Aramori I, Fukagawa M, Tsumura M, Iwami M, Ono H, Kojo H, Kohsaka M, Ueda Y, Imanaka H (1991c) Cloning and nucleotide sequencing of a novel 7 beta-(4-carboxybutanamido)cephalosporanic acid acylase gene of Bacillus laterosporus and its expression in Escherichia coli and Bacillus subtilis. J Bacteriol 173:7848–7855
Aramori I, Fukagawa M, Tsumura M, Iwami M, Ono H, Ishitani Y, Kojo H, Kohsaka M, Ueda Y, Imanaka H (1992) Comparative characterization of new glutaryl-7-ACA and cephalosporin C acylases. J Ferment Bioeng 73:185–192
Aretz W, Sauber K (1988) Novel d-amino acid transaminase. Ann NY Acad Sci 542:366–370
Burr KW, Ramsden M, Illing G, Harrison LA, Maishman NJ, Spence DW (1999) Industrial enzymes. US Patent 5,919,648
Cabri W, Verga R, Cambiaghi S, Bernasconi E (1999) Environmentally friendly production of 7-ACA. Chimica e Industria 81:461–464
Crawford MS (1991) One-step cephalosporin C amidase enzyme, a gene encoding the same, and expression thereof in a suitable host. EP Patent 0,405,846
Deshpande BS, Ambedkar SS, Shewale JG (1996) Cephalosporin C acylase and penicillin V acylase formation by Aeromonas sp. ACY 95. World J Microbiol Biotechnol 12:373–378
Duggleby HJ, Tolley SP, Hill CP, Dodson EJ, Dodson G, Moody PC (1995) Penicillin acylase has a single-amino-acid catalytic centre. Nature 373:264–268
Fechtig B, Peter H, Bickel H, Vischer E (1968) Concerning the preparation of 7-amino-cephalosporanic acid. Helv Chim Acta 51:1108–1119
Fritz-Wolf K, Koller KP, Lange G, Liesum A, Sauber K, Schreuder H, Aretz W, Kabsch W (2002) Structure-based prediction of modifications in glutarylamidase to allow single-step enzymatic production of 7-aminocephalosporanic acid from cephalosporin C. Protein Sci 11:92–103
Gaurav K, Kundu K, Kundu S (2010) Biosynthesis of cephalosporin-C acylase enzyme: optimal media design, purification, and characterization. Artif Cells Blood Substit Immobil Biotechnol 38:277–283
Harris CM, Ghisla S, Pollegioni L (2001) pH and kinetic effects in d-amino acid oxidase catalysis. Evidence for a concerted mechanism in substrate dehydrogenation via hydride transfer. Eur J Biochem 268:5504–5520
Ishii Y, Saito Y, Fujimura T, Sasaki H, Noguchi Y, Yamada H, Niwa M, Shimomura K (1995) High-level production, chemical modification and site-directed mutagenesis of a cephalosporin C acylase from Pseudomonas strain N176. Eur J Biochem 230:773–778
Kim Y, Hol WG (2001) Structure of cephalosporin acylase in complex with glutaryl-7-aminocephalosporanic acid and glutarate: insight into the basis of its substrate specificity. Chem Biol 8:1253–1264
Kim Y, Yoon K, Khang Y, Turley S, Hol WG (2000) The 2.0 A crystal structure of cephalosporin acylase. Structure 8:1059–1068
Kim Y, Kim S, Earnest TN, Hol WG (2002) Precursor structure of cephalosporin acylase. Insights into autoproteolytic activation in a new N-terminal hydrolase family. J Biol Chem 277:2823–2829
Kim JK, Yang IS, Rhee S, Dauter Z, Lee YS, Park SS, Kim KH (2003) Crystal structures of glutaryl 7-aminocephalosporanic acid acylase: insight into autoproteolytic activation. Biochemistry 42:4084–4093
Kim JK, Yang IS, Shin HJ, Cho KJ, Ryu EK, Kim SH, Park SS, Kim KH (2006) Insight into autoproteolytic activation from the structure of cephalosporin acylase: a protein with two proteolytic chemistries. Proc Natl Acad Sci U S A 103:1732–1737
Koller KP, Lange G, Sauber K (2002) Mutant glutaryl amidase and uses thereof. WO Patent 02/072806
Lein J (1988) One-step enzymatic conversion of cephalosporin C and derivatives to 7-aminocephalosporanic acid and derivatives. EP Patent 0,283,218
Lein J (1989) One-step enzymatic conversion of cephalosporin C and derivatives to 7-aminocephalosporanic acid and derivatives. EP Patent 0,322,032
Li Y, Chen J, Jiang W, Mao X, Zhao G, Wang E (1999) In vivo post-translational processing and subunit reconstitution of cephalosporin acylase from Pseudomonas sp. 130. Eur J Biochem 262:713–719
Lopez-Gallego F, Batencor L, Hidalgo A, Mateo C, Fernandez-Lafuente R, Guisan JM (2005) One-pot conversion of cephalosporin C to 7-aminocephalosporanic acid in the absence of hydrogen peroxide. Adv Synth Catal 347:1804–1810
Mao X, Wang W, Jiang W, Zhao GP (2004) His23beta and Glu455beta of the Pseudomonas sp. 130 glutaryl-7-amino cephalosporanic acid acylase are crucially important for efficient autoproteolysis and enzymatic catalysis. Protein J 23:197–204
Matsuda A, Matsuyama K, Yamamoto K, Ichikawa S, Komatsu KI (1987a) Cloning and characterization of the genes for two distinct cephalosporin acylases from a Pseudomonas strain. J Bacteriol 169:5815–5820
Matsuda A, Toma K, Komatsu KI (1987b) Nucleotide sequences of the genes for two distinct cephalosporin acylases from a Pseudomonas strain. J Bacteriol 169:5821–5829
Morin RB, Jackson BG, Flynn EH, Roseske RW, Andrews SL (1969) Chemistry of cephalosporin antibiotics XIV: reaction of cephalosporin C with nitrosyl chloride. J Am Chem Soc 91:1396–1400
Murzin AG, Brenner SE, Hubbard T, Chothia C (1995) SCOP: a structural classification of proteins database for the investigation of sequences and structures. J Mol Biol 247:536–540
Niwa M, Saito Y, Sasaki H, Ishii Y (1994) Cephalosporin C acylase. US Patent 5,336,613
Niwa M, Fujimura T, Ishii Y, Noguchi Y (1998) Cephalosporin C acylase. US Patent 5,804,429
Oh B, Kim M, Yoon J, Chung K, Shin Y, Lee D, Kim Y (2003) Deacylation activity of cephalosporin acylase to cephalosporin C is improved by changing the side-chain conformations of active-site residues. Biochem Biophys Res Commun 310:19–27
Oh B, Kim K, Park J, Yoon J, Han D, Kim Y (2004) Modifying the substrate specificity of penicillin G acylase to cephalosporin acylase by mutating active-site residues. Biochem Biophys Res Commun 319:486–492
Otten LG, Sio CF, Vrielink J, Cool RH, Quax WJ (2002) Altering the substrate specificity of cephalosporin acylase by directed evolution of the beta-subunit. J Biol Chem 277:42121–42127
Otten LG, Sio CF, van der Sloot AM, Cool RH, Quax WJ (2004) Mutational analysis of a key residue in the substrate specificity of a cephalosporin acylase. ChemBioChem 5:820–825
Otten LG, Sio CF, Reis CR, Koch G, Cool RH, Quax WJ (2007) A highly active adipyl-cephalosporin acylase obtained via rational randomization. FEBS J 274:5600–5610
Pilone MS, Pollegioni L (2002) D-amino acid oxidase as an industrial biocatalyst. Biocatal Biotrans 20:145–159
Pilone MS, Pollegioni L (2010) In: Flickinger MC (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation and cell technology. Wiley, New York, pp 1–11
Pilone MS, Buto S, Pollegioni L (1995) A process for bioconversion of cephalosporin C by Rhodotorula gracilis d-amino acid oxidase. Biotechnol Lett 17:199–204
Pollegioni L, Molla G (2011) New biotech applications from evolved d-amino acid oxidases. Trends Biotechnol 29:276–283
Pollegioni L, Lorenzi S, Rosini E, Marcone GL, Molla G, Verga R, Cabri W, Pilone MS (2005) Evolution of an acylase active on cephalosporin C. Protein Sci 14:3064–3076
Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G (2007) Physiological functions of d-amino acid oxidases: from yeast to humans. Cell Mol Life Sci 64:1373–1394
Pollegioni L, Molla G, Sacchi S, Rosini E, Verga R, Pilone MS (2008a) Properties and applications of microbial d-amino acid oxidases: current state and perspectives. Appl Microbiol Biotechnol 78:1–16
Pollegioni L, Pilone M, Molla G, Cucchetti E, Verga R, Cabri W (2008b) Cephalosporin C acylases. US Patent 7,399,624
Rosini E, Monelli CS, Pollegioni L, Riva S, Monti D (2012) On the substrate preference of glutaryl acylases. J Mol Catal B: Enzym 76:52–58
Saito Y, Fujimura T, Ishii Y, Noguchi Y, Miura T, Niwa M, Shimomura K (1996) Oxidative modification of a cephalosporin C acylase from Pseudomonas strain N176 and site-directed mutagenesis of the gene. Appl Environ Microbiol 62:2919–2925
Shin YC, Jeon JYJ, Jung KH, Park MR, Kim Y (2007) Cephalosporin C acylase mutant and method for preparing 7-ACA using same. US Patent 0,207,519
Sio CF, Riemens AM, van der Laan JM, Verhaert RM, Quax WJ (2002) Directed evolution of a glutaryl acylase into an adipyl acylase. Eur J Biochem 269:4495–4504
Sio CF, Otten LG, Cool RH, Quax WJ (2003) Analysis of a substrate specificity switch residue of cephalosporin acylase. Biochem Biophys Res Commun 312:755–760
Sio CF, Otten LG, Cool RH, Quax WJ (2006) Glutaryl amidases and their uses. US Patent 0,292,665
Sonawane VC (2006) Enzymatic modifications of cephalosporins by cephalosporin acylase and other enzymes. Crit Rev Biotechnol 26:95–120
Suzuki H, Miwa C, Ishihara S, Kumagai H (2004) A single amino acid substitution converts gamma-glutamyltranspeptidase to a class IV cephalosporin acylase (glutaryl-7-aminocephalosporanic acid acylase). Appl Environ Microbiol 70:6324–6328
Tischer W, Giesecke U, Lang G, Röder A, Wedekind F (1992) Biocatalytic 7-aminocephaIosporanic acid production. Ann NY Acad Sci 672:502–509
Volontè F, Marinelli F, Gastaldo L, Sacchi S, Pilone MS, Pollegioni L, Molla G (2008) Optimization of glutaryl-7-aminocephalosporanic acid acylase expression in E. coli. Protein Expr Purif 61:131–137
Volpato G, Rodrigues RC, Fernandez-Lafuente R (2010) Use of enzymes in the production of semi-synthetic penicillins and cephalosporins: drawbacks and perspectives. Curr Med Chem 17:3855–3873
Whang Y, Yu H, Song W, An M, Zhang J, Luo H, Shen Z (2012) Overexpression of synthesized cephalosporin C acylase containing mutations in the substrate transport tunnel. J Biosci Bioeng 113:36–41
Yamada H, Ishii Y, Noguchi Y, Miura T, Mori T, Saito Y (1996) Protein engineering of a cephalosporin C acylase. Ann NY Acad Sci 799:74–81
Yin J, Deng Z, Zhao G, Huang X (2011) The N-terminal nucleophile serine of cephalosporin acylase executes the second autoproteolytic cleavage and acylpeptide hydrolysis. J Biol Chem 286:24476–24486
Yoon JC, Choi SY (2010) Mutation cephalosporin C acylase. Korean Patent 10-2010-0056123
Zhu X, Luo H, Chang Y, Su H, Li Q, Yu H, Shen Z (2011) Characteristic of immobilizer cephalosporin C acylase and its application in one-step enzymatic conversion of cephalosporin C to 7-aminocephalosporanic acid. World J Microbial Biotechnol 27:823–829
Acknowledgments
We thank the support of Fondo di Ateneo per la Ricerca, Centro Grandi Attrezzature (Università dell’Insubria), and Consorzio Interuniversitario per le Biotecnologie. The authors are grateful to all members of their laboratory and particularly to Mirella Pilone.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pollegioni, L., Rosini, E. & Molla, G. Cephalosporin C acylase: dream and(/or) reality. Appl Microbiol Biotechnol 97, 2341–2355 (2013). https://doi.org/10.1007/s00253-013-4741-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4741-0