Abstract
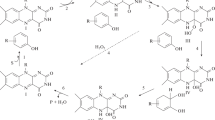
Thymol has antibacterial, antifungal, insecticidal, and antioxidative properties which are the basis for the wide use of this compound in the cosmetic, food, and pharmaceutical industries. Although thymol is a ubiquitously occurring substance in the environment, data about its degradation and detoxification by bacteria are sparse. Here, we show the existence of two different pathways for the biotransformation of thymol by Nocardia cyriacigeorgica and Mycobacterium neoaurum which were described for the first time for gram-positive bacteria. The first pathway starts with hydroxylation of thymol to thymohydroquinone (2-isopropyl-5-methylbenzene-1,4-diol) with subsequent oxidation to thymobenzoquinone (2-isopropyl-5-methyl-1,4-benzoquinone). The second pathway involves hydroxylation of the methyl group followed by oxidation to 3-hydroxy-4-isopropylbenzoic acid, possibly via the aldehyde 3-hydroxy-4-isopropylbenzaldehyde. It is noteworthy that the branched side chain of thymol was not oxidized. Similarities and differences of these oxidation processes with those of the gram-negative bacterium Pseudomonas putida, fungi, and plants are discussed and, in addition, the toxicity of thymol towards N. cyriacigeorgica and M. neoaurum was tested. The experiments showed a temporary growth inhibition with 0.025 % thymol. This was explained by degradation of thymol and the formation of products which are less toxic than thymol itself.




Similar content being viewed by others
References
Aeschbach R, Lögliger J, Scott BC, Murcia A, Butler J, Halliwell B, Aruoma OI (1994) Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Fd Chem Toxic 32:31–36
Ait-Ouazzou A, Cherrat L, Espina L, Loran S, Rota C, Pagan R (2011) The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Inn Food Sci Emerg Technol 12:320–329
Braga PC, Alfieri M, Culici M, Dal Sasso M (2007) Inhibitory activity of thymol against the formation and viability of Candida albicans hyphae. Mycoses 50:502–506
Chamberlain EM, Dagley S (1968) The metabolism of thymol by a Pseudomonas. Biochem J 110:755–763
Cosentino S, Tuberoso CIG, Pisano B, Satta M, Mascia V, Arzedi E, Palmas F (1999) In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett Appl Microbiol 29:130–135
Costa C, Lodesani M, Maistrello L (2010) Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 41:141–150
Daniel M (2006) Medicinal plants: chemistry and properties. Science, New Hampshire
De Boer TD, Backer HJ (1956) Diazomethane. In: Leonard NJ (ed) Organic synthesis, vol 36. Wiley, New York, pp 14–16
Derby R, Rohal P, Jackson C, Beutler A, Olsen C (2011) Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med 24:69–74
Didry N, Dubreuil L, Pinkas M (1994) Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm Act Helv 69:25–28
Fritsche W (1968) Effect of carbon sources on growth rate protein content and enzyme pattern in Candida guilliermondii. Z Allg Mikrobiol 8:91–99
Fritsche W, Hofrichter M (2005) Aerobic degradation of recalcitrant organic compounds by microorganisms. In: Jördening HJ, Winter J (eds) Environmental biotechnology. Wiley, Weinheim, pp 203–228
Ghasemi Y, Mohagheghzadeh A, Moshavash M, Ostovan Z, Rasoul-Amini S, Morowvat MH, Ghoshoon MB, Raee MJ, Mosavi-Azam SB (2009) Biotransformation of monoterpenes by Oocystis pusilla. World J Microbiol Biotechnol 25:1301–1304
Herter S, Mikolasch A, Schauer F (2012) Identification of phenylalkane derivatives when Mycobacterium neoaurum and Rhodococcus erythropolis were cultured in the presence of various phenylalkanes. Appl Microbiol Biotechnol 93:343–355
Hopper DJ, Chapman PJ (1971) Gentisic acid and its 3-methyl-substituted and 4-methyl-substituted homologues as intermediates in bacterial degradation of meta-cresol, 3,5-xylenol and 2,5-xylenol. Biochem J 122:19–28
Hundt K, Wagner M, Becher D, Hammer E, Schauer F (1998) Effect of selected environmental factors on degradation and mineralization of biaryl compounds by the bacterium Ralstonia picketii in soil and compost. Chemosphere 36:2321–2335
Kamel S, Brazier M, Desmet G, Fliniaux MA, Jacquin-Dubreuil A (1992) Glucosylation of butyric acid by cell-suspension culture of Nicotiana plumbaginifolia. Phytochem 31:1581–1583
Kreisel H, Schauer F (1987) Methoden des mykologischen Laboratoriums. Fischer, Stuttgart
Lagouri V, Blekas G, Tsimidou M, Kokkini S, Boskou D (1993) Composition and antioxidant activity of essential oils from oregano plants grown wild in Greece. Z Lebensm Unters Forsch 197:20–23
Nhi-Cong LT, Mikolasch A, Klenk HP, Schauer F (2009) Degradation of the multiple branched alkane 2,6,10,14-tetramethyl-pentadecane (pristane) in Rhodococcus ruber and Mycobacterium neoaurum. Int Biodeterior Biodegrad 63:201–207
Nhi-Cong LT, Mikolasch A, Awe S, Sheikhany H, Klenk HP, Schauer F (2010) Oxidation of aliphatic, branched chain, and aromatic hydrocarbons by Nocardia cyriacigeorgica isolated from oil-polluted sand samples collected in the Saudi Arabian Desert. J Basic Microbiol 50:241–253
Numpaque MA, Oviedo LA, Gil JH, Garcia CM, Durango DL (2011) Thymol and carvacrol: biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop Plant Pathol 36:3–13
Panda H (2000) Herbal cosmetics handbook. Asia Pacific Business Press, Delhi
Panda H (2002) The complete technology book on natural products (forest based). Asia Pacific Business Press, Delhi
Pandey SK, Upadhyay S, Tripathi AK (2009) Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) sprague seeds against Anopheles stephensi. Parasitol Res 105:507–512
Pinto E, Pina-Vaz C, Salgueiro L, Goncalves MJ, Costa-de-Oliveira S, Cavaleiro C, Palmeira A, Rodrigues A, Martinez-de-Oliveira J (2006) Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol 55:1367–1373
Poh CL, Bayly RC (1980) Evidence for isofunctional enzymes used in meta-cresol and 2,5-xylenol degradation via the gentisate pathway in Pseudomonas alcaligenes. J Bacteriol 143:59–69
Poulose AJ, Croteau R (1978) Biosynthesis of aromatic monoterpenes—conversion of gamma-terpinene to para-cymene and thymol in Thymus vulgaris L1. Arch Biochem Biophys 187:307–314
Samarasekera R, Weerasinghe IS, Hemalal KDP (2008) Insecticidal activity of menthol derivatives against mosquitoes. Pest Manag Sci 64:290–295
Scora RW (1967) Study of essential leaf oils of genus Monarda (Labiatae). Am J Bot 54:446–452
Shimoda K, Kondo Y, Nishida T, Hamada H, Nakajima N, Hamada H (2006) Biotransformation of thymol, carvacrol, and eugenol by cultured cells of Eucalyptus perriniana. Phytochem 67:2256–2261
Shrestha A, Rimal J, Rao A, Sequeira PS, Doshi D, Bhat GK (2011) In vitro antifungal effect of mouth rinses containing chlorhexidine and thymol. J Dent Sci 6:1–5
Ultee A, Bennik MHJ, Moezelaar R (2002) The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 68:1561–1568
Valero D, Valverde JM, Martinez-Romero A, Guillen F, Castillo S, Serrano M (2006) The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol Technol 41:317–327
Veldhuizen EJA, Tjeerdsma-Van Bokhoven JLM, Zweijtzer C, Burt SA, Haagsman HP (2006) Structural requirements for the antimicrobial activity of carvacrol. J Agr Food Chem 54:1874–1879
Vokou D, Kokkini S, Bessiere JM (1993) Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem Sys Ecol 21:287–295
Xu J, Zhou F, Ji BP, Pei RS, Xu N (2008) The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett Appl Microbiol 47:174–179
Acknowledgments
Robert Jack (Institute of Immunology, University of Greifswald) is gratefully acknowledged for help in preparing the manuscript. We thank M. Lalk (Institute of Pharmacy, University of Greifswald) for providing NMR data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hahn, V., Sünwoldt, K., Mikolasch, A. et al. Two different primary oxidation mechanisms during biotransformation of thymol by gram-positive bacteria of the genera Nocardia and Mycobacterium . Appl Microbiol Biotechnol 97, 1289–1297 (2013). https://doi.org/10.1007/s00253-012-4293-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4293-8