Abstract
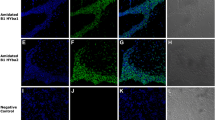
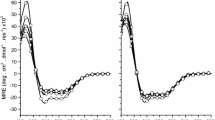
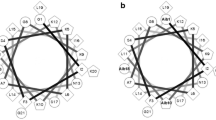
Many antimicrobial peptides from amphibian skin have been purified and structurally characterized and may be developed as therapeutic agents. Here we describe the antibacterial properties and membrane interaction of chensinin-1, a cationic arginine/histidine-rich antimicrobial peptide, from the skin secretions of Rana chensinensis. The amino acid composition, sequence, and atypical structure of chensinin-1 differ from other known antimicrobial peptides from amphibian skin. Chensinin-1 exhibited selective antimicrobial activity against Gram-positive bacteria, was inactive against Gram-negative bacteria, and had no hemolytic activity on human erythrocytes. The CD spectra for chensinin-1 indicated that the peptide adopted an aperiodic structure in water and a conformational structure with 20 % β-strands, 8 % α-helices, and the remaining majority of random coils in the trifluoroethanol or SDS solutions. Time-kill kinetics against Gram-positive Bacillus cereus demonstrated that chensinin-1 was rapidly bactericidal at 2× MIC and PAE was found to be >5 h. Chensinin-1 caused rapid and large dye leakage from negatively charged model vesicles. Furthermore, membrane permeation assays on intact B. cereus indicated that chensinin-1 induced membrane depolarization in less than 1 min and followed to damage the integrity of the cytoplasmic membrane and resulted in efflux of molecules from cytoplasma. Hence, the primary target of chensinin-1 action was the cytoplasmic membrane of bacteria. Chensinin-1 was unable to overcome bacterial resistance imposed by the lipopolysaccharide leaflet, the major constituent of the outer membrane of Gram-negative bacteria. Lipopolysaccharide induced oligomerization of chensinin-1, thus preventing its translocation across the outer membrane.








Similar content being viewed by others
References
Auvynet C, Rosenstein Y (2009) Multifunctional host defense peptides: antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J 276:6497–6508
Burkhart BM, Li N, Langs DA, Pangborn WA, Duax WL (1998) The conducting form of gramicidin A is a righthanded double-stranded double helix. Proc Natl Acad Sci USA 95:2950–12955
Chi SW, Kim JS, Kim DH, Lee SH, Park YH, Han KH (2007) Solution structure and membrane interaction mode of an antimicrobial peptide gaegurin 4. Biochem Biophys Res Commun 352:592–597
Conlon JM, Kolodziejek J, Nowotny N (2004) Antimicrobial peptides from ranidae frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim Biophys Acta 1696:1–14
Epand RF, Schmitt MA, Gellman SH, Epand RM (2006) Role of membrane lipids in the mechanism of bacterial species selective toxicity by two α/β-antimicrobial peptides. Biochim Biophys Acta 1758:1343–1350
Friedrich CL, Moyles D, Beveridge TJ, Hancock RE (2000) Antibacterial action of structurally diverse cationic peptides on Gram-positive bacteria. Antimicrob Agents Chemother 44:2086–2092
Ishibashi J, Sakanaka HS, Yang J, Sagisaka A, Yamakawa M (1999) Purification, cDNA cloning and modification of a defensin from the coconut rhinoceros beetle, Oryctes rhinoceros. Eur J Biochem 266:616–623
Jia Z (1997) Protein phosphatases: structures and implications. Biochem Cell Biol 75:7–26
Kan EJ, Demel RA, Bent A, Kruijff B (2003) The role of the abundant phenylalanines in the mode of action of the antimicrobial peptide clavanin. Biochim Biophys Acta 1615:84–92
Leptihn S, Har JY, Wohland TJ, Ding L (2010) Correlation of charge, hydrophobicity, and structure with antimicrobial activity of s1 and MIRIAM peptides. Biochemistry 49:9161–9170
Lequin O, Ladram A, Chabbert L, Bruston F, Conver O, Vanhoye D, Chassaing G, Nicolas P, Amiche M (2006) Dermaseptin S9, an α-helical antimicrobial peptide with a hydrophobic core and cationic termini. Biochemistry 45:468–480
Li L, He J, Eckert R, Yarbrough D, Lux R, Anderson M, Shi W (2010) Design and characterization of an acid-activated antimicrobial peptide. Chem Biol Drug Des 75:127–132
Li X, Feng W, Zhou M, Ma C, Chen T, Zeller M, Wang M, Shaw C (2011) Kasstasin: A novel potent vasoconstrictor peptide from the skin secretion of the African red-legged running frog Kassina maculata. Biochimie 93:1537–1542
Mangoni ML, Epand RF, Rosenfeld Y, Peleg A, Barra DR, Epand Y (2008) Lipopolysaccharide, a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization. J Biol Chem 283:22907–22917
Matsuzaki K, Murase O, Fujii N, Miyajima K (1996) An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 35:11361–11368
Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF (1988) Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem 263:7472–7477
Pál T, Abraham B, Sonnevend Á, Juma P, Conlon JM (2006) Brevinin-1BYa: a naturally occurring peptide from frog skin with broad-spectrum antibacterial and antifungal properties. Inter J Antimicrob Agents 27:525–529
Panchal RG, Smart ML, Bowser DN, Williams DA, Petrou S (2002) Pore-forming proteins and their application in biotechnology. Curr Pharm Biotechnol 3:99–115
Papo N, Shai Y (2005) A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides. J Biol Chem 280:10378–10387
Papo N, Oren Z, Pag U, Sahl HG, Shai Y (2002) The Consequence of sequence alteration of an amphipathic α-helical antimicrobial peptide and its diastereomers. J Biol Chem 277:33913–33921
Raghuraman H, Chattopadhyay A (2004) Interaction of melittin with membrane cholesterol: a fluorescence approach. Biophys J 87:2419–2432
Rasul R, Colea N, Balasubramanian D, Chen R, Kumar N, Willcox MDP (2010) Interaction of the antimicrobial peptide melimine with bacterial membranes. Inter J Antimicrob Agents 35:566–572
Rosenfeld Y, Barra D, Simmaco M, Shai Y, Mangoni ML (2006) A synergism between temporins toward Gram-negative bacteria overcomes resistance imposed by the lipopolysaccharide protective layer. J Biol Chem 281:28565–28574
Rozek A, Friedrich CL, Hancock RE (2000) Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 39:15765–15774
Sahl H, Pag GU, Bonness S, Wagner S, Antcheva N, Tossi A (2005) Mammalian defensins: structures and mechanism of antibiotic activity. J Leukocyte Biol 77:466–475
Sansom MS (1991) The biophysics of peptide models of ion channels. Prog Biophys Mol Biol 55:139–235
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462:55–70
Shang DJ, Yu FH, Li JF, Zheng JJ, Zhang LF, Li Y (2009) Molecular cloning of cDNAs encoding antimicrobial peptide precursors from the skin of the Chinese brown frog, Rana chensinensis. Zool Sci 26:220–226
Shi J, Ross CR, Chengappa MM, Blecha F (1994) Identification of a proline-arginine-rich antibacterial peptide from neutrophils that is analogous to PR-39, an antibacterial peptide from the small intestine. J Leukoc Biol 56:807–811
Sitaram N, Sai KP, Singh S, Sankaran K, Nagaraj R (2002) Structure-function relationship studies on the frog skin antimicrobial peptide tigerinin 1: design of analogs with improved activity and their action on clinical bacterial isolates. Antimicrob Agents Chemother 46:2279–2283
Takahashi D, Shukla SK, Prakash O, Zhang G (2010) Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 92:1236–1241
Tossi A, Sandri L, Giangaspero A (2000) Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4–30
Verdon J, Girardin N, Lacombe C, Berjeaud JM, Hechard Y (2009) δ-Hemolysin, an update on a membrane-interacting peptide. Peptides 30:817–823
Wu Y, Wang L, Zhou M, Ma C, Chen X, Bai B, Chen T, Shaw C (2011) Limnonectins: a new class of antimicrobial peptides from the skin secretion of the Fujian large-headed frog (Limnonectes fujianensis). Biochimie 93:981–987
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA 84:5449–5453
Zhang L, Dhillon P, Yan H, Farmer S, Hancock RE (2000) Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob Agents Chemother 44:317–3321
Acknowledgments
This work was supported by The National Natural Science Foundation of China (Grant No. 30970352), the Liaoning Key Laboratory Program (Grant No. 2008S131), and a Science Grant (2011E12SF031) from the Dalian Science and Technology Bureau.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 150 kb)
Rights and permissions
About this article
Cite this article
Shang, D., Sun, Y., Wang, C. et al. Membrane interaction and antibacterial properties of chensinin-1, an antimicrobial peptide with atypical structural features from the skin of Rana chensinensis . Appl Microbiol Biotechnol 96, 1551–1560 (2012). https://doi.org/10.1007/s00253-012-4148-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4148-3