Abstract
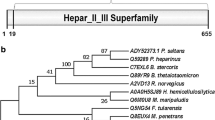
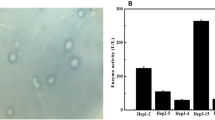
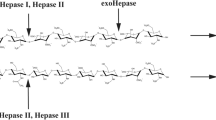
Recombinant heparinase III (rHepIII) from Bacteroides stercoris HJ-15 was cloned, expressed, and characterized. The full-length heparinase III gene from B. stercoris HJ-15 was identified by Southern blotting, and the sequence was deposited in GenBank. The heparinase III gene, which is 2,001-bp long, was cloned and overexpressed in Escherichia coli; highly active rHepIII was easily purified using only one step of immobilized Ni2+ affinity column chromatography. Enzymatic properties and substrate specificities of rHepIII were assessed, and its kinetic constants were calculated. rHepIII was most active in 50 mM sodium phosphate buffer with 350 mM NaCl (pH 6.6) at 45°C. Through amino acid modification studies and site-directed mutagenesis assay, cysteines and histidines were identified as crucial residues for enzymatic activity. Moreover, this enzyme digested not only heparan sulfate but also heparin and hyaluronic acid, and their degradation products were verified by strong anion exchange/high-performance liquid chromatography. These characteristics, including active residues and substrate specificities were interesting compared with those of existing heparinase III from other species. We anticipate that the convenience of purification and the characteristics of this enzyme will make it a powerful tool for studies of glycosaminoglycans and their lyases.




Similar content being viewed by others
References
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Conrad HE (1989) Structure of heparan sulfate and dermatan sulfate. Ann N Y Acad Sci 556:18–28
de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P (2007) Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc Natl Acad Sci U S A 104:19464–19469
Desai UR, Wang HM, Linhardt RJ (1993) Substrate specificity of the heparin lyases from Flavobacterium heparinum. Arch Biochem Biophys 306:461–468
Ernst S, Langer R, Cooney CL, Sasisekharan R (1995) Enzymatic degradation of glycosaminoglycans. Crit Rev Biochem Mol Biol 30:387–444
Gillet L, May JS, Stevenson PG (2009) In vivo importance of heparan sulfate-binding glycoproteins for murid herpesvirus-4 infection. J Gen Virol 90:602–613
Godavarti R, Davis M, Venkataraman G, Cooney C, Langer R, Sasisekharan R (1996) Heparinase III from Flavobacterium heparinum: cloning and recombinant expression in Escherichia coli. Biochem Biophys Res Commun 225:751–758
Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM (2009) Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol 83:2067–2074
Kim YS, Jo YY, Chang IM, Toida T, Park Y, Linhardt RJ (1996) A new glycosaminoglycan from the giant African snail Achatina fulica. J Biol Chem 271:11750–11755
Kim BT, Kim WS, Kim YS, Linhardt RJ, Kim DH (2000) Purification and characterization of a novel heparinase from Bacteroides stercoris HJ-15. J Biochem 128:323–328
Kjellen L, Lindahl U (1991) Proteoglycans: structures and interactions. Annu Rev Biochem 60:443–475
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lohse DL, Linhardt RJ (1992) Purification and characterization of heparin lyases from Flavobacterium heparinum. J Biol Chem 267:24347–24355
Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P (1992) Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol 12:240–247
Pojasek K, Shriver Z, Hu Y, Sasisekharan R (2000) Histidine 295 and histidine 510 are crucial for the enzymatic degradation of heparan sulfate by heparinase III. Biochemistry 39:4012–4019
Rapraeger AC (1993) The coordinated regulation of heparan sulfate, syndecans and cell behavior. Curr Opin Cell Biol 5:844–853
Rapraeger AC, Krufka A, Olwin BB (1991) Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 252:1705–1708
Rusnati M, Vicenzi E, Donalisio M, Oreste P, Landolfo S, Lembo D (2009) Sulfated K5 Escherichia coli polysaccharide derivatives: a novel class of candidate antiviral microbicides. Pharmacol Ther 123(3):310–322
Sasisekharan R, Moses MA, Nugent MA, Cooney CL, Langer R (1994) Heparinase inhibits neovascularization. Proc Natl Acad Sci U S A 91:1524–1528
Shilatifard A, Cummings RD (1995) Sulphate release from keratan sulphate and heparan sulphate by bovine Nacetylglucosamine-6-sulphate sulphatase. Glycobiology 5(3):291–297
Spear PG, Shieh MT, Herold BC, WuDunn D, Koshy TI (1992) Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv Exp Med Biol 313:341–353
Su H, Blain F, Musil RA, Zimmermann JJ, Gu K, Bennett DC (1996) Isolation and expression in Escherichia coli of hepB and hepC, genes coding for the glycosaminoglycan-degrading enzymes heparinase II and heparinase III, respectively, from Flavobacterium heparinum. Appl Environ Microbiol 62:2723–2734
Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM (1991) Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64:841–848
Zeisel MB, Barth H, Schuster C, Baumert TF (2009) Hepatitis C virus entry: molecular mechanisms and targets for antiviral therapy. Front Biosci 14:3274–3285
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hyun, YJ., Lee, J.H. & Kim, DH. Cloning, overexpression, and characterization of recombinant heparinase III from Bacteroides stercoris HJ-15. Appl Microbiol Biotechnol 86, 879–890 (2010). https://doi.org/10.1007/s00253-009-2327-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2327-7