Abstract
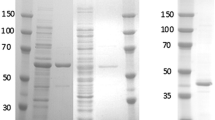
A cytoplasmic NADH oxidase (NOX) was purified from a soil bacteria, Brevibacterium sp. KU1309, which is able to grow in the medium containing 2-phenylethanol as the sole source of carbon under an aerobic condition. The enzyme catalyzed the oxidation of NADH to NAD+ involving two-electron reduction of O2 to H2O2. The molecular weight of the enzyme was estimated to be 102 kDa by gel filtration and 57 kDa by SDS-PAGE, which indicates that the NOX was a homodimer consisting of a single subunit. The enzyme was stable up to 70°C at a broad range of pH from 7 to 11. The enzyme activity increased about ten-fold with the addition of ammonium salt, while it was inhibited by Zn2+ (39%), Cu2+ (41%), Hg2+ (72%) and Ag+ (37%). The enzyme acts on NADH, but not on NADPH. The regeneration of NAD+ utilizing this enzyme made selective oxidation of mandelic acid or l-phenylalanine possible. This thermostable enzyme is expected to be applicable as a useful biocatalyst for NAD+ recycling.







Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Geueke B, Riebel B, Hummel W (2003) NADH oxidase from Lactobacillus brevis: a new catalyst for the regeneration of NAD. Enzyme and Microbial Technology 32:205–211
Ginson CM, Mallett TC, Claiborne AI, Caparon MG (2000) Contribution of NADH oxidase to aerobic metabolism of Streptococcus pyogenes. J Bacteriol 182:448–455
Herles C, Braune A, Blaut M (2002) Purification and characterization of an NADH oxidase from Eubacterium ramulus. Arch Microbiol 178:71–74
Higuchi M, Shimada M, Yamamoto Y, Hayashi T, Koga T, Kamio Y (1993) Identification of two distinct NADH oxidases corresponding to H2O2-forming oxidase and H2O-forming oxidase induced in Streptococcus mutans. J Gen Microbiol 139:2343–2351
Higuchi M, Shimada M, Matsumoto J, Yamamoto Y, Rhaman A, Kamio Y (1994) Molecular cloning and sequence analysis of the gene encoding the H2O2-forming NADH oxidase from Streptococcus mutans. Biosci Biotechnol Biochem 58:1603–1607
Hummel W, Riebel B (1996) Chiral alcohols by enantioselective enzymatic oxidation. Ann N Y Acad Sci 799:713–716
Hummel W, Riebel B (2003) Isolation and biochemical characterization of a new NADH oxidase from Lactobacillus brevis. Biotechnol Lett 25:51–54
Hummel W, Kuzu M, Geueke B (2003) An efficient and selective enzymatic oxidation system for the synthesis of enantiomerically pure d-tert-leucine. Organic Lett 5:3649–3650
Kawasaki S, Ishikura J, Chiba D, Nishino T, Niimura Y (2004) Purification and characterization of an H2O-forming NADH oxidase from Clostridium aminovalericum: existence of an oxygen-detoxifying enzyme in an obligate anaerobic bacteria. Arch Microbiol 181:324–330
Kengen SW, van der Oost J, de Vos WM (2003) Molecular characterization of H2O2-forming NADH oxidases from Archaeoglobus fulgidus. Eur J Biochem 270:2885–2894
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Maeda K, Truscott K, Liu XL, Scopes RK (1992) A thermostable NADH oxidase from anaerobic extreme thermophiles. Biochem J 284:551–555
Masullo M, Raimo G, Dello Russo A, Bocchini V, Bannister JV (1996) Purification and characterization of NADH oxidase from the archaea Sulfolobus acidocaldarius and Sulfolobus solfataricus. Biotechnol Appl Biochem 23:47–54
Matsumoto J, Higuchi M, Shimada M, Yamamoto Y, Kamio Y (1996) Molecular cloning and sequence analysis of the gene encoding the H2O-forming NADH oxidase from Streptococcus mutans. Biosci Biotechnol Biochem 60:39–43
Matsushita K, Otofuji A, Iwahashi M, Toyama H, Adachi O (2001) NADH dehydrogenase of Corynebacterium glutamicum. Purification of an NADH dehydrogenase II homolog able to oxidize NADPH. FEMS Microbiol Lett 204:271–276
Miyamoto K, Hirano J, Ohta H (2004) Efficient oxidation of alcohols by a 2-phenylethanol-degrading Brevibacterium sp. Biotechnol Lett 26:1385–1388
Miyoshi A, Rochat T, Gratadoux JJ, Le Loir Y, Oliveira SC, Langella P, Azevedo V (2003) Oxidative stress in Lactococcus lactis. Genet Mol Res 2:348–359
Odman P, Wellborn WB, Bommarius AS (2004) An enzymatic process to a-ketoglutarate from l-glutamate: the coupled system l-glutamate dehydrogenase/NADH oxidase. Tetrahedron Asymmetry 15:2933–2937
Park HJ, Reiser CO, Kondruweit S, Erdmann H, Schmid RD, Sprinzl M (1992) Purification and characterization of a NADH oxidase from the thermophile Thermus thermophilus HB8. Eur J Biochem 205:881–885
Riebel BR, Gibbs PR, Wellborn WB, Bommarius AS (2002) Cofactor regeneration of NAD+ from NADH: novel water-forming NADH oxidase. Adv Synth Catal 344:1156–1168
Riebel BR, Gibbs PR, Wellborn WB, Bommarius AS (2003) Cofactor regeneration of both NAD+ from NADH and NADP+ from NADPH:NADH oxidase from Lactobacillus sanfranciscensis. Adv Synth Catal 345:707–712
Ross RP, Claiborne A (1992) Molecular cloning and analysis of the gene encoding the NADH oxidase from Streptococcus faecalis 10C1. Comparison with NADH peroxidase and the flavoprotein disulfide reductases. J Mol Biol 227:658–671
Schmidt HL, Stocklein W, Danzer J, Kirch P, Limbach B (1986) Isolation and properties of an H2O-forming NADH oxidase from Streptococcus faecalis. Eur J Biochem 156:149–155
Tamura Y, Ohkubo A, Iwai S, Wada Y, Shinoda T, Arai K, Mineki S, Iida M, Taguchi H (2002) Two forms of NAD-dependent d-mandelate dehydrogenase in Enterococcus faecalis IAM 10071. Appl Environ Microbiol 68:947–951
Toomey D, Mayhew SG (1998) Purification and characterisation of NADH oxidase from Thermus aquaticus YT-1 and evidence that it functions in a peroxide-reduction system. Eur J Biochem 251:935–945
Yang X, Ma K (2005) Purification and characterization of an NADH oxidase from extremely thermophilic anaerobic bacterium Thermotoga hypogea. Arch Microbiol 183:331–337
Yang X, Ma K (2007) Characterization of an exceedingly active NADH oxidase from the anaerobic hyperthermophilic bacterium Thermotoga maritima. J Bacteriol 189:3312–3317
Acknowledgments
This work was partially supported by the Ministry of Education, Culture, Sports, Science and Technology, Grant-in-Aid for the 21st century COE Program entitled “Understanding and Control of Life’s Function via Systems Biology”, and Global COE program entitled “Center of Human Metabolomic Systems Biology” Keio University. The analysis of N-terminal amino acid sequence of NOX was assisted by Prof. Midori MATHUMOTO (Keio University), Associate Prof. Hiroyuki KAWAHARA (Hokkaido University), Mr. Tomohiro HIROSE (Hokkaido University), and Mr. Akira MIYAO (Hokkaido University), to whom we would like to express our thanks.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirano, Ji., Miyamoto, K. & Ohta, H. Purification and characterization of thermostable H2O2-forming NADH oxidase from 2-phenylethanol-assimilating Brevibacterium sp. KU1309. Appl Microbiol Biotechnol 80, 71–78 (2008). https://doi.org/10.1007/s00253-008-1535-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1535-x