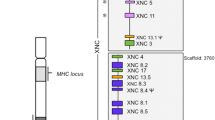
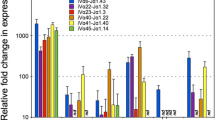
Abstract
Invariant T (iT) cells expressing an invariant or semi-invariant T cell receptor (TCR) repertoire have gained attention in recent years because of their potential as specialized regulators of immune function. These iT cells are typically restricted by nonclassical MHC class I molecules (e.g., CD1d and MR1) and undergo differentiation pathways distinct from conventional T cells. While the benefit of a limited TCR repertoire may appear counterintuitive in regard to the advantage of the diversified repertoire of conventional T cells allowing for exquisite specificity to antigens, the full biological importance and evolutionary conservation of iT cells are just starting to emerge. It is generally considered that iT cells are specialized to recognize conserved antigens equivalent to pathogen-associated molecular pattern. Until recently, little was known about the evolution of iT cells. The identification of class Ib and class I-like genes in nonmammalian vertebrates, despite the heterogeneity and variable numbers of these genes among species, suggests that iT cells are also present in ectothermic vertebrates. Indeed, recent studies in the amphibian Xenopus have revealed a drastic overrepresentation of several invariant TCRs in tadpoles and identified a prominent nonclassical MHC class I-restricted iT cell subset critical for tadpole antiviral immunity. This suggests an important and perhaps even dominant role of multiple nonclassical MHC class I-restricted iT cell populations in tadpoles and, by extension, other aquatic vertebrates with rapid external development that are under pressure to produce a functional lymphocyte repertoire with small numbers of cells.


Similar content being viewed by others
References
Anderson G, Moore NC, Owen JJ, Jenkinson EJ (1996) Cellular interactions in thymocyte development. Annu Rev Immunol 14:73–99
Barritt LC, Turpen JB (1995) Characterization of lineage restricted forms of a Xenopus CD45 homologue. Dev Comp Immunol 19:525–536
Bartl S, Baish MA, Flajnik MF, Ohta Y (1997) Identification of class I genes in cartilaginous fish, the most ancient group of vertebrates displaying an adaptive immune response. J Immunol 159:6097–6104
Bendelac A (1995) Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med 182:2091–2096
Bonnet M, Ferrier P, Spicuglia S (2009) Molecular genetics at the T-cell receptor beta locus: insights into the regulation of V(D)J recombination. Adv Exp Med Biol 650:116–132
Burrows SR, Chen Z, Archbold JK, Tynan FE, Beddoe T, Kjer-Nielsen L, Miles JJ, Khanna R, Moss DJ, Liu YC, Gras S, Kostenko L, Brennan RM, Clements CS, Brooks AG, Purcell AW, McCluskey J, Rossjohn J (2010) Hard wiring of T cell receptor specificity for the major histocompatibility complex is underpinned by TCR adaptability. Proc Natl Acad Sci U S A 107:10608–10613
Cheroutre H, Kronenberg M, Brorson K, Hunt SW 3rd, Eghtesady P, Hood L, Nickerson DA (1991) Analysis of MHC class I gene expression in adult bone marrow and fetal liver of the BALB/c mouse. J Immunol 146:3263–3272
Chida AS, Goyos A, Robert J (2011) Phylogenetic and developmental study of CD4, CD8 alpha and beta T cell co-receptor homologs in two amphibian species, Xenopus tropicalis and Xenopus laevis. Dev Comp Immunol 35:366–377
Chretien I, Robert J, Marcuz A, Garcia-Sanz JA, Courtet M, Du Pasquier L (1996) CTX, a novel molecule specifically expressed on the surface of cortical thymocytes in Xenopus. Eur J Immunol 26:780–791
Chretien I, Marcuz A, Fellah J, Charlemagne J, Du Pasquier L (1997) The T cell receptor beta genes of Xenopus. Eur J Immunol 27:763–771
Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, Nguyen H, Kang HS, Stanic AK, Joyce S, Koltun WA, Chorney MJ, Kronenberg M, Wang CR (2003) CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med 197:907–918
Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R (2005) Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol 174:3153–3157
Coles MC, Raulet DH (2000) NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4 + CD8+ cells. J Immunol 164:2412–2418
Cooper MD, Alder MN (2006) The evolution of adaptive immune systems. Cell 124:815–822
Dascher CC (2007) Evolutionary biology of CD1. Curr Top Microbiol Immunol 314:3–26
David-Watine B, Transy C, Gachelin G, Kourilsky P (1987) Tissue-specific expression of the mouse Q10 H-2 class-I gene during embryogenesis. Gene 61:145–154
Davis MM, Bjorkman PJ (1988) T-cell antigen receptor genes and T-cell recognition. Nature 334:395–402
De Jesus AF, Chen G, Li Z, Grayfer L, Robert J (2012) Susceptibility of Xenopus laevis tadpoles to infection by the ranavirus Frog-Virus 3 correlates with a reduced and delayed innate immune response in comparison with adult frogs. Virology 432:435–443
Dijkstra JM, Katagiri T, Hosomichi K, Yanagiya K, Inoko H, Ototake M, Aoki T, Hashimoto K, Shiina T (2007) A third broad lineage of major histocompatibility complex (MHC) class I in teleost fish; MHC class II linkage and processed genes. Immunogenetics 59:305–321
Du Pasquier L, Flajnik MF (1990) Expression of MHC class II antigens during Xenopus development. Dev Immunol 1:85–95
Du Pasquier L, Schwager J (1991) Immunoglobulin genes and B cell development in amphibians. Adv Exp Med Biol 292:1–9
Du Pasquier L, Weiss N (1973) The thymus during the ontogeny of the toad Xenopus laevis: growth, membrane-bound immunoglobulins and mixed lymphocyte reaction. Eur J Immunol 3:773–777
Du Pasquier L, Schwager J, Flajnik MF (1989) The immune system of Xenopus. Annu Rev Immunol 7:251–275
Du Pasquier L, Courtet M, Robert J (1995) A Xenopus lymphoid tumor cell line with complete Ig genes rearrangements and T-cell characteristics. Mol Immunol 32:583–593
Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117:1250–1259
Edholm ES, Albertorio Saez LM, Gill AL, Gill SR, Grayfer L, Haynes N, Myers JR, Robert J (2013) Nonclassical MHC class I-dependent invariant T cells are evolutionarily conserved and prominent from early development in amphibians. Proc Natl Acad Sci U S A 110:14342–14347
Edholm ES, Goyos A, Taran J, De JEsus AF, Ohta Y, Robert J (2014) Unusual evolutionary conservation and further species-specific adaptations of a large family of nonclassical MHC class Ib genes across different degrees of genome ploidy in the amphibian subfamily Xenopodinae. Immunogenetics. doi:10.1007/s0025101407745
Evans BJ (2008) Genome evolution and speciation genetics of clawed frogs (Xenopus and Silurana). Front Biosci 13:4687–4706
Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatella DC (2004) A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol Phylogenet Evol 33:197–213
Flajnik MF, Du Pasquier L, Cohen N (1985) Immune responses of thymus/lymphocyte embryonic chimeras: studies on tolerance and major histocompatibility complex restriction in Xenopus. Eur J Immunol 15:540–547
Flajnik MF, Kaufman JF, Hsu E, Manes M, Parisot R, Du Pasquier L (1986) Major histocompatibility complex-encoded class I molecules are absent in immunologically competent Xenopus before metamorphosis. J Immunol 137:3891–3899
Flajnik MF, Hsu E, Kaufman JF, Dupasquier L (1987) Changes in the immune-system during metamorphosis of Xenopus. Immunol Today 8:58–64
Flajnik MF, Kasahara M, Shum BP, Salter-Cid L, Taylor E, Du Pasquier L (1993) A novel type of class I gene organization in vertebrates: a large family of non-MHC-linked class I genes is expressed at the RNA level in the amphibian Xenopus. EMBO J 12:4385–4396
Gapin L, Matsuda JL, Surh CD, Kronenberg M (2001) NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol 2:971–978
Godfrey DI, Berzins SP (2007) Control points in NKT-cell development. Nat Rev Immunol 7:505–518
Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM (2010) Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8:e1000407
Gold MC, Eid T, Smyk-Pearson S, Eberling Y, Swarbrick GM, Langley SM, Streeter PR, Lewinsohn DA, Lewinsohn DM (2013) Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol 6:35–44
Goyos A, Guselnikov S, Chida AS, Sniderhan LF, Maggirwar SB, Nedelkovska H, Robert J (2007) Involvement of nonclassical MHC class Ib molecules in heat shock protein-mediated anti-tumor responses. Eur J Immunol 37:1494–1501
Goyos A, Ohta Y, Guselnikov S, Robert J (2009) Novel nonclassical MHC class Ib genes associated with CD8 T cell development and thymic tumors. Mol Immunol 46:1775–1786
Goyos A, Sowa J, Ohta Y, Robert J (2011) Remarkable conservation of distinct nonclassical MHC class I lineages in divergent amphibian species. J Immunol 186:372–381
Gravenor I, Horton TL, Ritchie P, Flint E, Horton JD (1995) Ontogeny and thymus-dependence of T cell surface antigens in Xenopus: flow cytometric studies on monoclonal antibody-stained thymus and spleen. Dev Comp Immunol 19:507–523
Grayfer L, Andino Fde J, Chen G, Chinchar GV, Robert J (2012) Immune evasion strategies of ranaviruses and innate immune responses to these emerging pathogens. Viruses 4:1075–1092
Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A (2007) Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity 27:751–762
Haire RN, Kitzan Haindfield MK, Turpen JB, Litman GW (2002) Structure and diversity of T-lymphocyte antigen receptors alpha and gamma in Xenopus. Immunogenetics 54:431–438
Hansen JD, Zapata AG (1998) Lymphocyte development in fish and amphibians. Immunol Rev 166:199–220
Haynes-Gilmore N, Banach M, Edholm ES, Lord E, Robert J (2014) A critical role of nonclassical MHC in tumor immune evasion in the amphibian Xenopus model. Carcinogenesis. doi:10.1093/carcin/bgu100
Hee CS, Gao S, Loll B, Miller MM, Uchanska-Ziegler B, Daumke O, Ziegler A (2010) Structure of a classical MHC class I molecule that binds “non-classical” ligands. PLoS Biol 8:e1000557
Hogquist KA, Baldwin TA, Jameson SC (2005) Central tolerance: learning self-control in the thymus. Nat Rev Immunol 5:772–782
Houlihan JM, Biro PA, Fergar-Payne A, Simpson KL, Holmes CH (1992) Evidence for the expression of non-HLA-A, -B, -C class I genes in the human fetal liver. J Immunol 149:668–675
Hunt DW, Huppertz HI, Jiang HJ, Petty RE (1994) Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood 84:4333–4343
Jameson SC, Hogquist KA, Bevan MJ (1995) Positive selection of thymocytes. Annu Rev Immunol 13:93–126
Jensen PE, Sullivan BA, Reed-Loisel LM, Weber DA (2004) Qa-1, a nonclassical class I histocompatibility molecule with roles in innate and adaptive immunity. Immunol Res 29:81–92
Jurgens JB, Gartland LA, Du Pasquier L, Horton JD, Gobel TW, Cooper MD (1995) Identification of a candidate CD5 homologue in the amphibian Xenopus laevis. J Immunol 155:4218–4223
Kau CL, Turpen JB (1983) Dual contribution of embryonic ventral blood island and dorsal lateral plate mesoderm during ontogeny of hemopoietic cells in Xenopus laevis. J Immunol 131:2262–2266
Kinjo Y, Kronenberg M (2005) V alpha14 i NKT cells are innate lymphocytes that participate in the immune response to diverse microbes. J Clin Immunol 25:522–533
Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491:717–723
Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A (2004) Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor alpha/delta locus. Immunol Rev 200:224–232
Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O (2010) Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11:701–708
Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O (2011) Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol 32:212–218
Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, Core M, Sleurs D, Serriari NE, Treiner E, Hivroz C, Sansonetti P, Gougeon ML, Soudais C, Lantz O (2013a) MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog 9:e1003681
Le Bourhis L, Mburu YK, Lantz O (2013b) MAIT cells, surveyors of a new class of antigen: development and functions. Curr Opin Immunol 25:174–180
Litman GW, Rast JP, Fugmann SD (2010) The origins of vertebrate adaptive immunity. Nat Rev Immunol 10:543–553
Lukacs MF, Harstad H, Bakke HG, Beetz-Sargent M, McKinnel L, Lubieniecki KP, Koop BF, Grimholt U (2010) Comprehensive analysis of MHC class I genes from the U-, S-, and Z-lineages in Atlantic salmon. BMC Genomics 11:154
Maniero GD, Morales H, Gantress J, Robert J (2006) Generation of a long-lasting, protective, and neutralizing antibody response to the ranavirus FV3 by the frog Xenopus. Dev Comp Immunol 30:649–657
Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, Cherif S, Vera G, Latour S, Soudais C, Lantz O (2009) Stepwise development of MAIT cells in mouse and human. PLoS Biol 7:e54
Michie AM, Zuniga-Pflucker JC (2002) Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin Immunol 14:311–323
Miller MM, Wang C, Parisini E, Coletta RD, Goto RM, Lee SY, Barral DC, Townes M, Roura-Mir C, Ford HL, Brenner MB, Dascher CC (2005) Characterization of two avian MHC-like genes reveals an ancient origin of the CD1 family. Proc Natl Acad Sci U S A 102:8674–8679
Morales HD, Robert J (2007) Characterization of primary and memory CD8 T-cell responses against ranavirus (FV3) in Xenopus laevis. J Virol 81:2240–2248
Mussmann R, Courtet M, Du Pasquier L (1998) Development of the early B cell population in Xenopus. Eur J Immunol 28:2947–2959
Nedelkovska H, Robert J (2012) Optimized transgenesis in Xenopus laevis/gilli isogenetic clones for immunological studies. Genesis 50:300–306
Nieuwkoop PD, Faber J (1967) Normal tables of Xenopus laevis (Daudin). In: Nieuwkoop PD (ed) A systematic and chronological survey of the development from the fertilized egg till the end of metamorphosis, 2nd edn. North-Holland, Amsterdam, pp 162–188
Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, Reantragoon R, Sandoval-Romero ML, Sullivan LC, Brooks AG, Chen Z, Fairlie DP, McCluskey J, Rossjohn J (2013) Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun 4:2142
Prince AL, Yin CC, Enos ME, Felices M, Berg LJ (2009) The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunol Rev 228:115–131
Robert J, Cohen N (1998) Ontogeny of CTX expression in Xenopus. Dev Comp Immunol 22:605–612
Robert J, Cohen N (1999) In vitro differentiation of a CD4/CD8 double-positive equivalent thymocyte subset in adult Xenopus. Int Immunol 11:499–508
Robert J, Gregory Chinchar V (2012) “Ranaviruses: an emerging threat to ectothermic vertebrates” report of the First International Symposium on Ranaviruses, Minneapolis MN July 8, 2011. Dev Comp Immunol 36:259–261
Robert J, Sung M, Cohen N (2001) In vitro thymocyte differentiation in MHC class I-negative Xenopus larvae. Dev Comp Immunol 25:323–336
Rollins-Smith LA (1998) Metamorphosis and the amphibian immune system. Immunol Rev 166:221–230
Rollins-Smith LA, Barker KS, Davis AT (1997a) Involvement of glucocorticoids in the reorganization of the amphibian immune system at metamorphosis. Dev Immunol 5:145–152
Rollins-Smith LA, Flajnik MF, Blair PJ, Davis AT, Green WF (1997b) Involvement of thyroid hormones in the expression of MHC class I antigens during ontogeny in Xenopus. Dev Immunol 5:133–144
Rollins-Smith LA, Davis AT, Reinert LK (2000) Pituitary involvement in T cell renewal during development and metamorphosis of Xenopus laevis. Brain Behav Immun 14:185–197
Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI (2012) Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol 12:845–857
Rothenberg EV, Taghon T (2005) Molecular genetics of T cell development. Annu Rev Immunol 23:601–649
Rudolph MG, Stanfield RL, Wilson IA (2006) How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol 24:419–466
Salomonsen J, Sorensen MR, Marston DA, Rogers SL, Collen T, van Hateren A, Smith AL, Beal RK, Skjodt K, Kaufman J (2005) Two CD1 genes map to the chicken MHC, indicating that CD1 genes are ancient and likely to have been present in the primordial MHC. Proc Natl Acad Sci U S A 102:8668–8673
Salter-Cid L, Nonaka M, Flajnik MF (1998) Expression of MHC class Ia and class Ib during ontogeny: high expression in epithelia and coregulation of class Ia and lmp7 genes. J Immunol 160:2853–2861
Sammut B, Laurens V, Tournefier A (1997) Isolation of MHC class I cDNAs from the axolotl Ambystoma mexicanum. Immunogenetics 45:285–294
Sammut B, Du Pasquier L, Ducoroy P, Laurens V, Marcuz A, Tournefier A (1999) Axolotl MHC architecture and polymorphism. Eur J Immunol 29:2897–2907
Schwager J, Burckert N, Courtet M, Du Pasquier L (1989) Genetic basis of the antibody repertoire in Xenopus: analysis of the Vh diversity. EMBO J 8:2989–3001
Schwager J, Burckert N, Courtet M, Du Pasquier L (1991) The ontogeny of diversification at the immunoglobulin heavy chain locus in Xenopus. EMBO J 10:2461–2470
Sidobre S, Kronenberg M (2002) CD1 tetramers: a powerful tool for the analysis of glycolipid-reactive T cells. J Immunol Methods 268:107–121
Simoni Y, Diana J, Ghazarian L, Beaudoin L, Lehuen A (2013) Therapeutic manipulation of natural killer (NK) T cells in autoimmunity: are we close to reality? Clin Exp Immunol 171:8–19
Skold M, Behar SM (2003) Role of CD1d-restricted NKT cells in microbial immunity. Infect Immun 71:5447–5455
Star B, Nederbragt AJ, Jentoft S, Grimholt U, Malmstrom M, Gregers TF, Rounge TB, Paulsen J, Solbakken MH, Sharma A, Wetten OF, Lanzen A, Winer R, Knight J, Vogel JH, Aken B, Andersen O, Lagesen K, Tooming-Klunderud A, Edvardsen RB, Tina KG, Espelund M, Nepal C, Previti C, Karlsen BO, Moum T, Skage M, Berg PR, Gjoen T, Kuhl H, Thorsen J, Malde K, Reinhardt R, Du L, Johansen SD, Searle S, Lien S, Nilsen F, Jonassen I, Omholt SW, Stenseth NC, Jakobsen KS (2011) The genome sequence of Atlantic cod reveals a unique immune system. Nature 477:207–210
Starr TK, Jameson SC, Hogquist KA (2003) Positive and negative selection of T cells. Annu Rev Immunol 21:139–176
Tochinai S (1980) Direct observation of cell migration into Xenopus thymus rudiments through mesenchyme. Dev Comp Immunol 4:273–282
Treiner E (2003) MAIT lymphocytes, regulators of intestinal immunity? Press Med 32:1636–1637
Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422:164–169
Tsukamoto K, Deakin JE, Graves JA, Hashimoto K (2013) Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics 65:115–124
Turpen JB, Smith PB (1989) Precursor immigration and thymocyte succession during larval development and metamorphosis in Xenopus. J Immunol 142:41–47
Urdahl KB, Sun JC, Bevan MJ (2002) Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol 3:772–779
Wang C, Perera TV, Ford HL, Dascher CC (2003) Characterization of a divergent non-classical MHC class I gene in sharks. Immunogenetics 55:57–61
Wilson SB, Delovitch TL (2003) Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol 3:211–222
Wu L, Gabriel CL, Parekh VV, Van Kaer L (2009) Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities. Tissue Antigens 73:535–545
Zon LI, Mather C, Burgess S, Bolce ME, Harland RM, Orkin SH (1991) Expression of GATA-binding proteins during embryonic development in Xenopus laevis. Proc Natl Acad Sci U S A 88:10642–10646
Acknowledgments
We would like to thank Drs. Leon Grayfer and Nikesha Haynes-Gilmore for helpful discussions and critical reading of the manuscript. This research was supported by grants R24-AI-059830 from the National Institute of Allergy and Infectious Diseases (NIH/NIAID).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robert, J., Edholm, ES. A prominent role for invariant T cells in the amphibian Xenopus laevis tadpoles. Immunogenetics 66, 513–523 (2014). https://doi.org/10.1007/s00251-014-0781-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-014-0781-6