Abstract
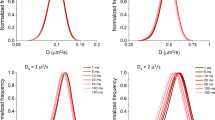
During eccentric contraction, muscle is lengthening so that the actin-myosin cross-bridges bear a load that exceeds the force they generate during isometric contraction. Using the optical trap technique, we simulated eccentric contraction at the single molecule level and investigated the effect of load on the skeletal actomyosin lifetime at different ATP concentrations. The range of the loads was up to 17 pN above the isometric level. We found that the frequency distribution of the lifetime of the actin-bound state of the myosin molecule was biphasic: it quickly rose and then decreased slowly. The rate of the slow phase of this distribution increased with both the load and the ATP concentration. The fast phase accelerated sharply with the load, but it was independent of ATP concentration. The presence of the fast phase demonstrates that some transition(s) in the actomyosin complex occur before the myosin head becomes able to bind ATP and detach from actin. Its high sensitivity to the load indicates that the transition is load-dependent.



Similar content being viewed by others
References
Bell GI (1978) Models for the specific adhesion of cells to cells. Science 200:618–627
Bickham DC, West TG, Webb MR, Woledge RC, Curtin NA, Ferenczi MA (2011) Millisecond-scale biochemical response to change in strain. Biophys J 101:2445–2454
Brunello E, Reconditi M, Elangovan R, Linari M, Sun YB, Narayanan T, Panine P, Piazzesi G, Irving M, Lombardi V (2007) Skeletal muscle resists stretch by rapid binding of the second motor domain of myosin to actin. Proc Natl Acad Sci USA 104:20114–20119
Capitanio M, Canepari M, Maffei M, Beneventi D, Monico C, Vanzi F, Bottinelli R, Pavone FS (2012) Ultrafast force-clamp spectroscopy of single molecules reveals load dependence of myosin working stroke. Nat Methods 9:1013–1019
Cavagna GA, Citterio G (1974) Effect of stretching on the elastic characteristics and the contractile component of frog striated muscle. J Physiol 239:1–14
Colombini B, Bagni MA, Romano G, Cecchi G (2007) Characterization of actomyosin bond properties in intact skeletal muscle by force spectroscopy. Proc Natl Acad Sci USA 104:9284–9289
Ferenczi MA, Bershitsky SY, Koubassova NA, Kopylova GV, Fernandez M, Narayanan T, Tsaturyan AK (2014) Why muscle is an efficient shock absorber. PLoS One. doi:10.1371/journal.pone.0085739
Finer JT, Simmons RM, Spudich JA (1994) Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368:113–119
Greenberg MJ, Lin T, Goldman YE, Shuman H, Ostap EM (2012) Myosin IC generates power over a range of loads via a new tension-sensing mechanism. Proc Natl Acad Sci USA 109:E2433–E2440
Linari M, Wolegde RC, Curtin NA (2003) Energy storage during stretch of active single fibres from frog skeletal muscle. J Physiol 548:461–474
Linari M, Caremani M, Piperio C, Brandt P, Lombardi V (2007) Stiffness and fraction of myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys J 92:2476–2490
Lombardi V, Piazzesi G (1990) The contractile response during steady lengthening of stimulated frog muscle fibres. J Physiol 431:141–171
Lymn RW, Taylor EW (1971) Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10:4617–4624
Margossian SS, Lowey S (1982) Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol 85:55–71
Molloy JE, Burns JE, Kendrick-Jones J, Tregear RT, White DC (1995) Movement and force produced by a single myosin head. Nature 378:209–212
Nabiev SR, Ovsyannikov DA, Bershitsky BY, Bershitsky SY (2008) Optical trap as a tool for studying motor proteins. Biofizika 53:929–935 (in Russian)
Nishizaka T, Miyata H, Yoshikawa H, Ishiwata S, Kinosita K (1995) Unbinding force of a single motor molecule of muscle measured using optical tweezers. Nature 377:251–254
Pardee JD, Spudich JA (1982) Purification of muscle actin. Methods Enzymol 85:164–181
Piazzesi G, Lucii L, Lombardi V (2002) The size and the speed of the working stroke of muscle myosin and its dependence on the force. J Physiol 545:145–151
Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V (2007) Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131:784–795
Svoboda K, Block SM (1994) Biological applications of optical forces. Annu Rev Biophys Biomol Struct 23:247–285
Takagi Y, Homsher EE, Goldman YE, Shuman H (2006) Force generation in single conventional actomyosin complexes under high dynamic load. Biophys J 90:1295–1307
Thirlwell H, Corrie JE, Reid JP, Trentham DR, Ferenczi MA (1994) Kinetics of relaxation from rigor of permeabilized fast-twitch skeletal fibers from the rabbit using a novel caged ATP and apyrase. Biophys J 67:2436–2447
Tsaturyan AK, Bershitsky SY, Koubassova NA, Fernandez M, Narayanan T, Ferenczi MA (2011) The fraction of myosin motors that participate in isometric contraction of rabbit muscle fibers at near-physiological temperature. Biophys J 101:404–410
Veigel C, Bartoo ML, White DC, Sparrow JC, Molloy JE (1998) The stiffness of rabbit skeletal actomyosin cross-bridges determined with an optical tweezers transducer. Biophys J 75:1424–1438
Veigel C, Molloy JE, Schmitz S, Kendrick-Jones J (2003) Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat Cell Biol 5:980–986
Acknowledgments
The authors are grateful to Dr. Oleg Lookin for providing software of single event analysis and Dr. Timothy West for careful reading of the manuscript and useful comments. The work was supported by RFBR grants 13-04-40101-N (to SB) and 13-04-40100-N (to AT) and by the Program of the RAS Ural Branch (project 12-P-4-1007 to SB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nabiev, S.R., Ovsyannikov, D.A., Tsaturyan, A.K. et al. The lifetime of the actomyosin complex in vitro under load corresponding to stretch of contracting muscle. Eur Biophys J 44, 457–463 (2015). https://doi.org/10.1007/s00249-015-1048-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-015-1048-3