Abstract
The goods and services provided by riverine systems are critical to humanity, and our reliance increases with our growing population and demands. As our activities expand, these systems continue to degrade throughout the world even as we try to restore them, and many efforts have not met expectations. One way to increase restoration effectiveness could be to explicitly design restorations to promote microbial communities, which are responsible for much of the organic matter breakdown, nutrient removal or transformation, pollutant removal, and biomass production in river ecosystems. In this paper, we discuss several design concepts that purposefully create conditions for these various microbial goods and services, and allow microbes to act as ecological restoration engineers. Focusing on microbial diversity and function could improve restoration effectiveness and overall ecosystem resilience to the stressors that caused the need for the restoration. Advances in next-generation sequencing now allow the use of microbial ‘omics techniques (e.g., metagenomics, metatranscriptomics) to assess stream ecological conditions in similar fashion to fish and benthic macroinvertebrates. Using representative microbial communities from stream sediments, biofilms, and the water column may greatly advance assessment capabilities. Microbes can assess restorations and ecosystem function where animals may not currently be present, and thus may serve as diagnostics for the suitability of animal reintroductions. Emerging applications such as ecological metatranscriptomics may further advance our understanding of the roles of specific restoration designs towards ecological services as well as assess restoration effectiveness.

Similar content being viewed by others
References
Albert JS, Destouni G, Duke-Sylvester SM et al (2021) Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 50:85–94. https://doi.org/10.1007/s13280-020-01318-8
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163. https://doi.org/10.1017/S1464793105006950
Reid AJ, Carlson AK, Creed IF et al (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Harding JS, Benfield EF, Bolstad PV et al (1998) Stream biodiversity: the ghost of land use past. Proc Natl Acad Sci 95:14843–14847. https://doi.org/10.1073/pnas.95.25.14843
Walter RC, Merritts DJ (2008) Natural streams and the legacy of water-powered mills. Science 319:299–304. https://doi.org/10.1126/science.1151716
Kaushal SS, Groffman PM, Mayer PM, Striz E, Gold AJ (2008) Effects of stream restoration on denitrification in an urbanizing watershed. Ecol Applic 18:789–804. https://doi.org/10.1890/07-1159.1
Tian Z, Zhao H, Peter KT, Gonzalez M, Wetzel J, Wu C, Hu X, Prat J, Mudrock E, Hettinger R, Cortina AE (2021) A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science 371(6525):185–189. https://doi.org/10.1126/science.abd6951
Moore J, Fanelli RM, Sekellick AJ (2020) High-frequency data reveal deicing salts drive elevated specific conductance and chloride along with pervasive and frequent exceedances of the US Environmental Protection Agency aquatic life criteria for chloride in urban streams. Environ Sci Technol 54:778–789. https://doi.org/10.1021/acs.est.9b04316
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol Soc 24:706–723. https://doi.org/10.1899/04-028.1
Utz RM, Hilderbrand RH, Boward DM (2009) Identifying regional differences in threshold responses of aquatic invertebrates to land cover gradients. Ecol Indic 9:556–567. https://doi.org/10.1016/j.ecolind.2008.08.008
Utz RM, Hilderbrand RH, Raesly RL (2010) Regional differences in patterns of fish species loss with changing land use. Biol Conserv 143:688–699. https://doi.org/10.1016/j.biocon.2009.12.006
Hilderbrand RH, Utz RM (2015) Ecological thresholds and resilience in streams. In: Rowiński P, Radecki-Pawlik A (eds) Rivers – physical, fluvial and environmental processes. Springer International Publishing, Cham, pp 461–478
Bernhardt ES, Palmer MA, Allan JD et al (2005) Synthesizing U.S. river restoration efforts. Science 308:636–637. https://doi.org/10.1126/science.1109769
Stranko SA, Hilderbrand RH, Palmer MA (2012) Comparing the fish and benthic macroinvertebrate diversity of restored urban streams to reference streams. Restor Ecol 20:747–755. https://doi.org/10.1111/j.1526-100X.2011.00824.x
Violin CR, Cada P, Sudduth EB et al (2011) Effects of urbanization and urban stream restoration on the physical and biological structure of stream ecosystems. Ecol Appl 21:1932–1949
Fanelli RM, Prestegaard KL, Palmer MA (2019) Urban legacies: aquatic stressors and low aquatic biodiversity persist despite implementation of regenerative stormwater conveyance systems. Freshw Sci 38:818–833. https://doi.org/10.1086/706072
dos Reis Oliveira PC, van der Geest HG, Kraak MHS et al (2020) Over forty years of lowland stream restoration: lessons learned? J Environ Manage 264:110417. https://doi.org/10.1016/j.jenvman.2020.110417
Palmer MA, Filoso S, Fanelli RM (2014) From ecosystems to ecosystem services: stream restoration as ecological engineering. Ecol Eng 65:62–70. https://doi.org/10.1016/j.ecoleng.2013.07.059
Lüderitz V, Speierl T, Langheinrich U et al (2011) Restoration of the Upper Main and Rodach rivers – the success and its measurement. Ecol Eng 37:2044–2055. https://doi.org/10.1016/j.ecoleng.2011.07.010
Findlay S (2010) Stream microbial ecology. J North Am Benthol Soc 29:170–181. https://doi.org/10.1899/09-023.1
Cassán F, Coniglio A, López G et al (2020) Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol Fertil Soils 56:461–479. https://doi.org/10.1007/s00374-020-01463-y
Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N (2014) Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Factories 13:66. https://doi.org/10.1186/1475-2859-13-66
Patil PL, Medhane NS (1974) Seed inoculation studies in gram (Cicer arietinum L.) with different strains of Rhizobium sp. Plant Soil 40:221–223. https://doi.org/10.1007/BF00011425
Venkataraman GS, Neelakantan S (1967) Effect of the cellular constituents of the nitrogen-fixing blue-green alga, Cylindrospermum muscicola, on the root growth of rice plants. J Gen Appl Microbiol 13:53–61. https://doi.org/10.2323/jgam.13.53
Harris J (2009) Soil microbial communities and restoration ecology: facilitators or followers? Science 325:573–574. https://doi.org/10.1126/science.1172975
Singh Rawat V, Kaur J, Bhagwat S et al (2022) Deploying microbes as drivers and indicators in ecological restoration. Restor Ecol. https://doi.org/10.1111/rec.13688
Yan X, Wang J, Hu X et al (2021) Contrasting effects of microbial fertiliser and organic fertiliser on soil bacterial community in coal mine dump of Inner Mongolia. Chem Ecol 37:384–398. https://doi.org/10.1080/02757540.2021.1886283
Su H, Lin J, Chen H, Wang Q (2021) Production of a novel slow-release coal fly ash microbial fertilizer for restoration of mine vegetation. Waste Manag 124:185–194. https://doi.org/10.1016/j.wasman.2021.02.007
Hou D, O’Connor D, Igalavithana AD et al (2020) Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat Rev Earth Environ 1:366–381. https://doi.org/10.1038/s43017-020-0061-y
Patel AB, Shaikh S, Jain KR et al (2020) Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front Microbiol 11:562813. https://doi.org/10.3389/fmicb.2020.562813
Emery SM, Rudgers JA (2011) Beach restoration efforts influenced by plant variety, soil inoculum, and site effects. J Coast Res 27:636–644. https://doi.org/10.2112/JCOASTRES-D-10-00120.1
Crawford KM, Busch MH, Locke H, Luecke NC (2020) Native soil microbial amendments generate trade-offs in plant productivity, diversity, and soil stability in coastal dune restorations. Restor Ecol 28:328–336. https://doi.org/10.1111/rec.13073
Luecke NC, Mejia AJ, Crawford KM (2021) Native soil amendments combined with commercial arbuscular mycorrhizal fungi increase biomass of Panicum amarum. Sci Rep 11:17865. https://doi.org/10.1038/s41598-021-97307-2
Ohsowski BM, Klironomos JN, Dunfield KE, Hart MM (2012) The potential of soil amendments for restoring severely disturbed grasslands. Appl Soil Ecol 60:77–83. https://doi.org/10.1016/j.apsoil.2012.02.006
Koziol L, Crews TE, Bever JD (2020) Native plant abundance, diversity, and richness increases in prairie restoration with field inoculation density of native mycorrhizal amendments. Restor Ecol 28. https://doi.org/10.1111/rec.13151
Middleton EL, Richardson S, Koziol L et al (2015) Locally adapted arbuscular mycorrhizal fungi improve vigor and resistance to herbivory of native prairie plant species. Ecosphere 6:art276. https://doi.org/10.1890/ES15-00152.1
Cheeke TE, Schneider M, Saify A et al (2022) Role of soil biota in grassland restorations in high nutrient soils. Restor Ecol 30. https://doi.org/10.1111/rec.13549
Cai J-F, Jiang F, Liu X-S et al (2021) Biochar-amended coastal wetland soil enhances growth of Suaeda salsa and alters rhizosphere soil nutrients and microbial communities. Sci Total Environ 788:147707. https://doi.org/10.1016/j.scitotenv.2021.147707
Chavarria KA, Saltonstall K, Vinda J et al (2021) Land use influences stream bacterial communities in lowland tropical watersheds. Sci Rep 11:21752. https://doi.org/10.1038/s41598-021-01193-7
Palmer MA, Ambrose RF, Poff NL (1997) Ecological theory and community restoration ecology. Restor Ecol 5:291–300. https://doi.org/10.1046/j.1526-100X.1997.00543.x
Brown T, Berg J, Underwood K (2010) Replacing incised headwater channels and failing stormwater infrastructure with regenerative stormwater conveyance. Low impact development 2010. American Society of Civil Engineers, San Francisco, California, United States, pp 207–217
United States Environmental Protection Agency Stormwater Phase II Final Rule: Small MS4 Stormwater Program Overview. U S Environmental Protection Agency, Office of Water, Washington DC.
Cizek AR, Hunt WF, Winston RJ et al (2018) Water quality and hydrologic performance of a regenerative stormwater conveyance in the Piedmont of North Carolina. J Environ Eng 144:04018062. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001344
Filoso S, Palmer MA (2011) Assessing stream restoration effectiveness at reducing nitrogen export to downstream waters. Ecol Appl 21:1989–2006
Koryto KM, Hunt WF, Page JL (2017) Hydrologic and water quality performance of regenerative stormwater conveyance installed to stabilize an eroded outfall. Ecol Eng 108:263–276. https://doi.org/10.1016/j.ecoleng.2017.04.041
Duan S, Mayer PM, Kaushal SS et al (2019) Regenerative stormwater conveyance (RSC) for reducing nutrients in urban stormwater runoff depends upon carbon quantity and quality. Sci Total Environ 652:134–146. https://doi.org/10.1016/j.scitotenv.2018.10.197
Duan S, Newcomer-Johnson T, Mayer P, Kaushal S (2016) Phosphorus retention in stormwater control structures across streamflow in urban and suburban watersheds. Water 8:390. https://doi.org/10.3390/w8090390
McClain ME, Boyer EW, Dent CL et al (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312
Craig L, Bahr JM, Roden EE (2010) Localized zones of denitrification in a floodplain aquifer in southern Wisconsin, USA. Hydrogeol J 18:1867–1879. https://doi.org/10.1007/s10040-010-0665-2
Freixa A, Ejarque E, Crognale S et al (2016) Sediment microbial communities rely on different dissolved organic matter sources along a Mediterranean river continuum: DOM utilization by river sediments. Limnol Oceanogr 61:1389–1405. https://doi.org/10.1002/lno.10308
Stegen JC, Fredrickson JK, Wilkins MJ et al (2016) Groundwater–surface water mixing shifts ecological assembly processes and stimulates organic carbon turnover. Nat Commun 7:11237. https://doi.org/10.1038/ncomms11237
Wei Z, Liu Y, Feng K et al (2018) The divergence between fungal and bacterial communities in seasonal and spatial variations of wastewater treatment plants. Sci Total Environ 628–629:969–978. https://doi.org/10.1016/j.scitotenv.2018.02.003
Naegeli MW, Uehlinger U (1997) Contribution of the hyporheic zone to ecosystem metabolism in a prealpine gravel-bed-river. J North Am Benthol Soc 16:794–804. https://doi.org/10.2307/1468172
Battin TJ, Kaplan LA, Newbold JD, Hendricks SP (2003) A mixing model analysis of stream solute dynamics and the contribution of a hyporheic zone to ecosystem function*: hyporheic zone DOC dynamics. Freshw Biol 48:995–1014. https://doi.org/10.1046/j.1365-2427.2003.01062.x
Boulton AJ (2007) Hyporheic rehabilitation in rivers: restoring vertical connectivity. Freshw Biol 52:632–650. https://doi.org/10.1111/j.1365-2427.2006.01710.x
Boulton AJ, Datry T, Kasahara T et al (2010) Ecology and management of the hyporheic zone: stream–groundwater interactions of running waters and their floodplains. J North Am Benthol Soc 29:26–40. https://doi.org/10.1899/08-017.1
Edwards RT (1987) Sestonic bacteria as a food source for filtering invertebrates in two southeastern blackwater rivers1: Seston in blackwater rivers. Limnol Oceanogr 32:221–234. https://doi.org/10.4319/lo.1987.32.1.0221
Wiegner TN, Kaplan LA, Newbold JD, Ostrom PH (2005) Contribution of dissolved organic C to stream metabolism: a mesocosm study using 13 C-enriched tree-tissue leachate. J North Am Benthol Soc 24:48–67.
Lautz LK, Fanelli RM (2008) Seasonal biogeochemical hotspots in the streambed around restoration structures. Biogeochemistry 91:85–104. https://doi.org/10.1007/s10533-008-9235-2
Hester ET, Doyle MW (2008) In-stream geomorphic structures as drivers of hyporheic exchange: in-stream structures and hyporheic exchange. Water Resour Res 44. https://doi.org/10.1029/2006WR005810
Lautz LK, Siegel DI (2006) Modeling surface and ground water mixing in the hyporheic zone using MODFLOW and MT3D. Adv Water Resour 29:1618–1633. https://doi.org/10.1016/j.advwatres.2005.12.003
Fanelli RM, Lautz LK (2008) Patterns of water, heat, and solute flux through streambeds around small dams. Groundwater 46:671–687
Kasahara T, Hill AR (2006) Hyporheic exchange flows induced by constructed riffles and steps in lowland streams in southern Ontario, Canada. Hydrol Process 20:4287–4305. https://doi.org/10.1002/hyp.6174
Serrana JM, Li B, Sumi T et al (2021) Profiling the microbial community structure and functional diversity of a dam-regulated river undergoing gravel bar restoration. Freshw Biol 66:2170–2184. https://doi.org/10.1111/fwb.13824
Aldridge KT, Brookes JD, Ganf GG (2009) Rehabilitation of stream ecosystem functions through the reintroduction of coarse particulate organic matter. Restor Ecol 17:97–106. https://doi.org/10.1111/j.1526-100X.2007.00338.x
Brugger A, Wett B, Kolar I et al (2001) Immobilization and bacterial utilization of dissolved organic carbon entering the riparian zone of the alpine Enns River, Austria. Aquat Microb Ecol 24:129–142. https://doi.org/10.3354/ame024129
Findlay S, Tank J, Dye S et al (2002) A cross-system comparison of bacterial and fungal biomass in detritus pools of headwater streams. Microb Ecol 43:55–66. https://doi.org/10.1007/s00248-001-1020-x
Flores L, Díez JR, Larrañaga A et al (2013) Effects of retention site on breakdown of organic matter in a mountain stream. Freshw Biol 58:1267–1278. https://doi.org/10.1111/fwb.12125
Merz JE, Ochikubo Chan LK (2005) Effects of gravel augmentation on macroinvertebrate assemblages in a regulated California river. River Research and Applications 21:61–74. https://doi.org/10.1002/rra.819
Ouellet V, Daniels MD, Peipoch M et al (2022) Beyond the light effect: how hydrologic and geomorphologic stream features control microbial distribution across pool sequences in a temperate headwater stream. Ecohydrology 15:e2380. https://doi.org/10.1002/eco.2380
Battin TJ, Besemer K, Bengtsson MM et al (2016) The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol 14:251–263. https://doi.org/10.1038/nrmicro.2016.15
Singer G, Besemer K, Schmitt-Kopplin P et al (2010) Physical heterogeneity increases biofilm resource use and its molecular diversity in stream mesocosms. PLoS One 5:e9988. https://doi.org/10.1371/journal.pone.0009988
Noe GB, Boomer K, Gillespie JL et al (2019) The effects of restored hydrologic connectivity on floodplain trapping vs. release of phosphorus, nitrogen, and sediment along the Pocomoke River. Maryland USA. Ecol Eng 138:334–352. https://doi.org/10.1016/j.ecoleng.2019.08.002
dos Santos M, Pinto R, Weigelhofer G, Diaz-Pines E et al (2020) River-floodplain restoration and hydrological effects on GHG emissions: biogeochemical dynamics in the parafluvial zone. Sci Total Environ 715:136980. https://doi.org/10.1016/j.scitotenv.2020.136980
Preiner S, Bondar-Kunze E, Pitzl B et al (2020) Effect of hydrological connectivity on the phosphorus buffering capacity of an urban floodplain. Front Environ Sci 8:147. https://doi.org/10.3389/fenvs.2020.00147
Holling CS, Meffe GK (1996) Command and control and the pathology of natural resource management. Conserv Biol 10:328–337. https://doi.org/10.1046/j.1523-1739.1996.10020328.x
Lear G, Washington V, Neale M et al (2013) The biogeography of stream bacteria: the biogeography of stream bacteria. Glob Ecol Biogeogr 22:544–554. https://doi.org/10.1111/geb.12046
Hug LA, Baker BJ, Anantharaman K et al (2016) A new view of the tree of life. Nat Microbiol 1:16048. https://doi.org/10.1038/nmicrobiol.2016.48
Washington VJ, Lear G, Neale MW, Lewis GD (2013) Environmental effects on biofilm bacterial communities: a comparison of natural and anthropogenic factors in New Zealand streams. Freshw Biol. https://doi.org/10.1111/fwb.12208
Good SP, Urycki DR, Crump BC (2018) Predicting Hydrologic function with aquatic gene fragments. Water Resour Res 54:2424–2435. https://doi.org/10.1002/2017WR021974
Urycki DR, Bassiouni M, Good SP et al (2022) The streamwater microbiome encodes hydrologic data across scales. Sci Total Environ 849:157911. https://doi.org/10.1016/j.scitotenv.2022.157911
Simonin M, Voss KA, Hassett BA et al (2019) In search of microbial indicator taxa: shifts in stream bacterial communities along an urbanization gradient. Environ Microbiol 21:3653–3668. https://doi.org/10.1111/1462-2920.14694
Lowe RL (1974) Environmental requirements and pollution tolerance of freshwater diatoms. Environmental Protection Agency, National Environmental Research Center, Office of Research and Development, U.S
van Dam H, Mertens A, Sinkeldam J (1994) A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherland J Aquat Ecol 28:117–133. https://doi.org/10.1007/BF02334251
Carlisle D, Meador MR, Short TM, et al (2013) The quality of our Nation’s waters--ecological health in the Nation’s streams, 1993-2005. U.S. Geological Survey Circular 1391, 120 p. http://pubs.usgs.gov/circ/1391/. Accessed 14 October 2022.
Potapova M, Carlisle D (2011) Development and application of indices to assess the condition of benthic algal communities in U.S. streams and rivers. U.S. Geological Survey Open File Report 2011-1126, 40 p. https://pubs.usgs.gov/of/2011/1126/ofr2011-1126.pdf. Accessed 14 October 2022.
Porter SD, Mueller DK, Spahr NE et al (2008) Efficacy of algal metrics for assessing nutrient and organic enrichment in flowing waters. Freshw Biol 53:1036–1054. https://doi.org/10.1111/j.1365-2427.2007.01951.x
Rimet F, Abarca N, Bouchez A et al (2018) The potential of high-throughput sequencing (HTS) of natural samples as a source of primary taxonomic information for reference libraries of diatom barcodes. Fottea 18:37–54. https://doi.org/10.5507/fot.2017.013
Rimet F, Gusev E, Kahlert M et al (2019) Diat.barcode, an open-access curated barcode library for diatoms. Sci Rep 9:15116. https://doi.org/10.1038/s41598-019-51500-6
Smucker NJ, Pilgrim EM, Nietch CT et al (2020) DNA metabarcoding effectively quantifies diatom responses to nutrients in streams. Ecol Appl 30:e02205. https://doi.org/10.1002/eap.2205
Hagy JD, Devereux R, Houghton KA, et al (2018) Developing microbial community indicators of nutrient exposure in southeast coastal plain streams using a molecular approach. US Environmental Protection Agency, Office of Research and Development, National Health and Environmental Effects Research Laboratory, Research Triangle Park, NC. EPA 600/R-17/490. 44 pp.
Hagy JD, Houghton KA, Beddick DL et al (2020) Quantifying stream periphyton assemblage responses to nutrient amendments with a molecular approach. Freshw Sci 39:292–308. https://doi.org/10.1086/708935
Salis RK, Bruder A, Piggott JJ et al (2017) High-throughput amplicon sequencing and stream benthic bacteria: identifying the best taxonomic level for multiple-stressor research. Sci Rep 7:44657. https://doi.org/10.1038/srep44657
Hilderbrand RH, Keller SR, Laperriere SM et al (2020) Microbial communities can predict the ecological condition of headwater streams. PLOS One 15:e0236932. https://doi.org/10.1371/journal.pone.0236932
Lau KEM, Washington VJ, Fan V et al (2015) A novel bacterial community index to assess stream ecological health. Freshw Biol 60:1988–2002. https://doi.org/10.1111/fwb.12625
Niu L, Li Y, Wang P et al (2018) Development of a microbial community-based index of biotic integrity (MC-IBI) for the assessment of ecological status of rivers in the Taihu Basin, China. Ecol Indic 85:204–213. https://doi.org/10.1016/j.ecolind.2017.10.051
Li J, Li Y, Qian B et al (2017) Development and validation of a bacteria-based index of biotic integrity for assessing the ecological status of urban rivers: a case study of Qinhuai River basin in Nanjing, China. J Environ Manage 196:161–167. https://doi.org/10.1016/j.jenvman.2017.03.003
Laperriere SM, Hilderbrand RH, Keller SR et al (2020) Headwater stream microbial diversity and function across agricultural and urban land use gradients. Appl Environ Microbiol 86:e00018–e00020. https://doi.org/10.1128/AEM.00018-20
Crump BC, Amaral-Zettler LA, Kling GW (2012) Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J 6:1629–1639. https://doi.org/10.1038/ismej.2012.9
Hosen JD, Febria CM, Crump BC, Palmer MA (2017) Watershed urbanization linked to differences in stream bacterial community composition. Front Microbiol 8:1452. https://doi.org/10.3389/fmicb.2017.01452
Urycki DR, Good SP, Crump BC et al (2020) River microbiome composition reflects macroscale climatic and geomorphic differences in headwater streams. Front Water 2:43. https://doi.org/10.3389/frwa.2020.574728
Walsh CJ, Fletcher TD, Ladson AR (2005) Stream restoration in urban catchments through redesigning stormwater systems: looking to the catchment to save the stream. J North Am Benthol Soc 24:690–705. https://doi.org/10.1899/04-020.1
King RS, Baker ME (2010) Considerations for analyzing ecological community thresholds in response to anthropogenic environmental gradients. J North Am Benthol Soc 29:998–1008. https://doi.org/10.1899/09-144.1
Lear G, Lewis GD (2009) Impact of catchment land use on bacterial communities within stream biofilms. Ecol Indic 9:848–855. https://doi.org/10.1016/j.ecolind.2008.10.001
Lear G, Anderson MJ, Smith JP et al (2008) Spatial and temporal heterogeneity of the bacterial communities in stream epilithic biofilms: heterogeneity of bacteria in stream biofilms. FEMS Microbiol Ecol 65:463–473. https://doi.org/10.1111/j.1574-6941.2008.00548.x
Karr JR (1981) Assessment of biotic integrity using fish communities. Fisheries 6:21–27.
Southerland MT, Rogers GM, Kline MJ et al (2007) Improving biological indicators to better assess the condition of streams. Ecol Indic 7:751–767. https://doi.org/10.1016/j.ecolind.2006.08.005
Merritt RW, Cummins KW (1996) An introduction to the aquatic insects of North America3rd edn. Kendall/Hunt Pub Co, Dubuque, Iowa
Hempel CA, Wright N, Harvie J et al (2022) Metagenomics versus total RNA sequencing: most accurate data-processing tools, microbial identification accuracy and perspectives for ecological assessments. Nucleic Acids Res 50:9279–9293. https://doi.org/10.1093/nar/gkac689
Maurya S, Abraham JS, Somasundaram S et al (2020) Indicators for assessment of soil quality: a mini-review. Environ Monit Assess 192:604. https://doi.org/10.1007/s10661-020-08556-z
Crump BC, Wojahn JM, Tomas F, Mueller RS (2018) Metatranscriptomics and amplicon sequencing reveal mutualisms in seagrass microbiomes. Front Microbiol 9:388. https://doi.org/10.3389/fmicb.2018.00388
Satinsky BM, Crump BC, Smith CB et al (2014) Microspatial gene expression patterns in the Amazon River Plume. Proc Natl Acad Sci 111:11085–11090. https://doi.org/10.1073/pnas.1402782111
Acknowledgements
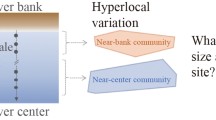
The University of Maryland Center for Environmental Science, Integration and Application Network provided the symbol libraries to create Figure 1.
Funding
This work was supported by Chesapeake Bay Trust Pooled Monitoring Initiative Restoration Research award 19724 to RHH and by National Science Foundation awards DEB-1840243 and EAR-1836768 to TB and BCC.
Author information
Authors and Affiliations
Contributions
Robert H. Hilderbrand conceived of the manuscript. Robert H. Hilderbrand, Ted Bambakidis, and Byron C. Crump conducted the literature review and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hilderbrand, R.H., Bambakidis, T. & Crump, B.C. The Roles of Microbes in Stream Restorations. Microb Ecol 85, 853–861 (2023). https://doi.org/10.1007/s00248-023-02179-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02179-w