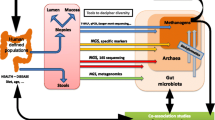
Abstract
Methanogens are microorganisms belonging to the Archaea domain and represent the primary source of biotic methane. Methanogens encode a series of enzymes which can convert secondary substrates into methane following three major methanogenesis pathways. Initially recognized as environmental microorganisms, methanogens have more recently been acknowledged as host-associated microorganisms after their detection and initial isolation in ruminants in the 1950s. Methanogens have also been co-detected with bacteria in various pathological situations, bringing their role as pathogens into question. Here, we review reported associations between methanogens and bacteria in physiological and pathological situations in order to understand the metabolic interactions explaining these associations. To do so, we describe the origin of the metabolites used for methanogenesis and highlight the central role of methanogens in the syntrophic process during carbon cycling. We then focus on the metabolic abilities of co-detected bacterial species described in the literature and infer from their genomes the probable mechanisms of their association with methanogens. The syntrophic interactions between bacteria and methanogens are paramount to gut homeostasis. Therefore, any dysbiosis affecting methanogens might impact human health. Thus, the monitoring of methanogens may be used as a bio-indicator of dysbiosis. Moreover, new therapeutic approaches can be developed based on their administration as probiotics. We thus insist on the importance of investigating methanogens in clinical microbiology.






Similar content being viewed by others
Data Availability
All the genomes analyzed in this review were retrieved from the NCBI Nucleotide database. Accession numbers are available in the supplementary material.
Code Availability
Not applicable.
References
Hug LA, Baker BJ, Anantharaman K, Brown CT, Probst AJ, Castelle CJ et al (2016) A new view of the tree of life. Nat Microbiol 1:16048
Tidjani Alou M, Naud S, Khelaifia S, Bonnet M, Lagier J-C, Raoult D. State of the art in the culture of the human microbiota: new interests and strategies. Clin Microbiol Rev. 2020;34.
Woolhouse M, Scott F, Hudson Z, Howey R, Chase-Topping M (2012) Human viruses: discovery and emergence. Philos Trans R Soc Lond B Biol Sci 367:2864–2871
Heintz-Buschart A, Wilmes P (2018) Human gut microbiome: function matters. Trends Microbiol 26:563–574
Koskinen K, Pausan MR, Perras AK, Beck M, Bang C, Mora M, et al. First insights into the diverse human archaeome: specific detection of archaea in the gastrointestinal tract, lung, and nose and on skin. mBio. 2017;8.
Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PloS One. 2009;4:e7063.
Samuel BS, Gordon JI (2006) A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A 103:10011–10016
Ruaud A, Esquivel-Elizondo S, de la Cuesta-Zuluaga J, Waters JL, Angenent LT, Youngblut ND, et al. Syntrophy via interspecies H2 transfer between Christensenella and Methanobrevibacter underlies their global cooccurrence in the human gut. mBio. 2020;11.
Robert C, Del’Homme C, Bernalier-Donadille A. Interspecies H2 transfer in cellulose degradation between fibrolytic bacteria and H2-utilizing microorganisms from the human colon. FEMS Microbiol Lett. 2001;205:209–14.
Sieber JR, McInerney MJ, Gunsalus RP (2012) Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Annu Rev Microbiol 66:429–452
Mihajlovski A, Alric M, Brugère J-F (2008) A putative new order of methanogenic archaea inhabiting the human gut, as revealed by molecular analyses of the mcrA gene. Res Microbiol 159:516–521
Nottingham PM, Hungate RE (1968) Isolation of methanogenic bacteria from feces of man. J Bacteriol 96:2178–2179
Smith PH, Hungate RE (1958) Isolation and characterization of Methanobacterium ruminantium n. sp. J Bacteriol 75:713–718
Lovley DR, Greening RC, Ferry JG (1984) Rapidly growing rumen methanogenic organism that synthesizes coenzyme M and has a high affinity for formate. Appl Environ Microbiol 48:81–87
Miller TL, Wolin MJ, Conway de Macario E, Macario AJ (1982) Isolation of Methanobrevibacter smithii from human feces. Appl Environ Microbiol 43:227–232
Miller TL, Wolin MJ (1983) Oxidation of hydrogen and reduction of methanol to methane is the sole energy source for a methanogen isolated from human feces. J Bacteriol 153:1051–1055
Miller TL, Wolin MJ (1985) Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol 141:116–122
van de Wijngaard WM, Creemers J, Vogels GD, van der Drift C (1991) Methanogenic pathways in Methanosphaera stadtmanae. FEMS Microbiol Lett 64:207–211
Oxley APA, Lanfranconi MP, Würdemann D, Ott S, Schreiber S, McGenity TJ et al (2010) Halophilic archaea in the human intestinal mucosa. Environ Microbiol 12:2398–2410
Khelaifia S, Raoult D, Drancourt M. A versatile medium for cultivating methanogenic archaea.PloS One. 2013;8:e61563.
Khelaifia S, Garibal M, Robert C, Raoult D, Drancourt M. Draft genome sequence of a human-associated isolate of Methanobrevibacter arboriphilicus, the lowest-G+C-content archaeon. Genome Announc. 2014;2.
Nava GM, Carbonero F, Croix JA, Greenberg E, Gaskins HR (2012) Abundance and diversity of mucosa-associated hydrogenotrophic microbes in the healthy human colon. ISME J 6:57–70
Dridi B, Fardeau ML, Ollivier B, Raoult D, Drancourt M (2012) Methanomassiliicoccus luminyensis gen nov sp nov a methanogenic archaeon isolated from human faeces. Int J Syst Evol Microbiol 62(1902):7
Gorlas A, Robert C, Gimenez G, Drancourt M, Raoult D (2012) Complete genome sequence of Methanomassiliicoccus luminyensis, the largest genome of a human-associated archaea species. J Bacteriol 194:4745
Dridi B, Henry M, Richet H, Raoult D, Drancourt M (2012) Age-related prevalence of Methanomassiliicoccus luminyensis in the human gut microbiome. APMIS Acta Pathol Microbiol Immunol Scand 120:773–777
Borrel G, Harris HMB, Tottey W, Mihajlovski A, Parisot N, Peyretaillade E et al (2012) Genome sequence of “Candidatus Methanomethylophilus alvus” Mx1201, a methanogenic archaeon from the human gut belonging to a seventh order of methanogens. J Bacteriol 194:6944–6945
Borrel G, Harris HMB, Parisot N, Gaci N, Tottey W, Mihajlovski A, et al. Genome sequence of “Candidatus Methanomassiliicoccus intestinalis” Issoire-Mx1, a third Thermoplasmatales-related methanogenic archaeon from human feces. Genome Announc. 2013;1.
Keppler F, Schiller A, Ehehalt R, Greule M, Hartmann J, Polag D. Stable isotope and high precision concentration measurements confirm that all humans produce and exhale methane. J Breath Res. 2016;10:016003.
Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177.
Mihajlovski A, Doré J, Levenez F, Alric M, Brugère J-F (2010) Molecular evaluation of the human gut methanogenic archaeal microbiota reveals an age-associated increase of the diversity. Environ Microbiol Rep 2:272–280
Wampach L, Heintz-Buschart A, Hogan A, Muller EEL, Narayanasamy S, Laczny CC et al (2017) Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front Microbiol 8:738
Rani SB, Balamurugan R, Ramakrishna BS (2017) Molecular analysis of the human faecal archaea in a southern Indian population. J Biosci 42:113–119
Grine G, Boualam MA, Drancourt M (2017) Methanobrevibacter smithii, a methanogen consistently colonising the newborn stomach. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 36:2449–2455
Miller TL, Wolin MJ (1982) Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol 131:14–18
Hudson MJ, Tomkins AM, Wiggins HS, Drasar BS (1993) Breath methane excretion and intestinal methanogenesis in children and adults in rural Nigeria. Scand J Gastroenterol 28:993–998
Stewart JA, Chadwick VS, Murray A (2006) Carriage, quantification, and predominance of methanogens and sulfate-reducing bacteria in faecal samples. Lett Appl Microbiol 43:58–63
Brusa T, Conca R, Ferrara A, Ferrari A, Pecchioni A (1987) The presence of methanobacteria in human subgingival plaque. J Clin Periodontol 14:470–471
Belay N, Johnson R, Rajagopal BS, Conway de Macario E, Daniels L (1988) Methanogenic bacteria from human dental plaque. Appl Environ Microbiol 54:600–3
Brusa T, Canzi E, Allievi L, Del Puppo E, Ferrari A (1993) Methanogens in the human intestinal tract and oral cavity. Curr Microbiol 27:261–265
Ferrari A, Brusa T, Rutili A, Canzi E, Biavati B (1994) Isolation and characterization of Methanobrevibacter oralis sp nov. Curr Microbiol 29(7):12
Grine G, Terrer E, Boualam MA, Aboudharam G, Chaudet H, Ruimy R et al (2018) Tobacco-smoking-related prevalence of methanogens in the oral fluid microbiota. Sci Rep 8:9197
Huynh HTT, Nkamga VD, Signoli M, Tzortzis S, Pinguet R, Audoly G et al (2016) Restricted diversity of dental calculus methanogens over five centuries. France Sci Rep 6:25775
Manzoor S, Schnürer A, Bongcam-Rudloff E, Müller B (2016) Complete genome sequence of Methanoculleus bourgensis strain MAB1, the syntrophic partner of mesophilic acetate-oxidising bacteria (SAOB). Stand Genomic Sci 11:80
Achtman M, Zhou Z (2020) Metagenomics of the modern and historical human oral microbiome with phylogenetic studies on Streptococcus mutans and Streptococcus sobrinus. Philos Trans R Soc Lond B Biol Sci 375:20190573
Ward TL, Hosid S, Ioshikhes I, Altosaar I (2013) Human milk metagenome: a functional capacity analysis. BMC Microbiol 13:116
Togo AH, Grine G, Khelaifia S, des Robert C, Brevaut V, Caputo A, et al. Culture of methanogenic archaea from human colostrum and milk. Sci Rep. 2019;9:18653.
Hassani Y, Brégeon F, Aboudharam G, Drancourt M, Grine G. Detection of Methanobrevobacter smithii and Methanobrevibacter oralis in lower respiratory tract microbiota. Microorganisms. 2020;8.
Million M, Tidjani Alou M, Khelaifia S, Bachar D, Lagier J-C, Dione N et al (2016) Increased gut redox and depletion of anaerobic and methanogenic prokaryotes in severe acute malnutrition. Sci Rep 6:26051
Tidjani Alou M, Million M, Traore SI, Mouelhi D, Khelaifia S, Bachar D et al (2017) Gut bacteria missing in severe acute malnutrition, can we identify potential probiotics by culturomics? Front Microbiol 8:899
Camara A, Konate S, Tidjani Alou M, Kodio A, Togo AH, Cortaredona S et al (2021) Clinical evidence of the role of Methanobrevibacter smithii in severe acute malnutrition. Sci Rep 11:5426
Matarazzo F, Ribeiro AC, Feres M, Faveri M, Mayer MPA (2011) Diversity and quantitative analysis of archaea in aggressive periodontitis and periodontally healthy subjects. J Clin Periodontol 38:621–627
Kulik EM, Sandmeier H, Hinni K, Meyer J (2001) Identification of archaeal rDNA from subgingival dental plaque by PCR amplification and sequence analysis. FEMS Microbiol Lett 196:129–133
Lepp PW, Brinig MM, Ouverney CC, Palm K, Armitage GC, Relman DA (2004) Methanogenic archaea and human periodontal disease. Proc Natl Acad Sci U S A 101:6176–6181
Robichaux M, Howell M, Boopathy R (2003) Methanogenic activity in human periodontal pocket. Curr Microbiol 46:53–58
Yamabe K, Maeda H, Kokeguchi S, Tanimoto I, Sonoi N, Asakawa S et al (2008) Distribution of archaea in Japanese patients with periodontitis and humoral immune response to the components. FEMS Microbiol Lett 287:69–75
Vianna ME, Holtgraewe S, Seyfarth I, Conrads G, Horz HP (2008) Quantitative analysis of three hydrogenotrophic microbial groups, methanogenic archaea, sulfate-reducing bacteria, and acetogenic bacteria, within plaque biofilms associated with human periodontal disease. J Bacteriol 190:3779–3785
Vianna ME, Conrads G, Gomes BPFA, Horz HP (2009) T-RFLP-based mcrA gene analysis of methanogenic archaea in association with oral infections and evidence of a novel Methanobrevibacter phylotype. Oral Microbiol Immunol 24:417–422
Li CL, Liu DL, Jiang YT, Zhou YB, Zhang MZ, Jiang W et al (2009) Prevalence and molecular diversity of archaea in subgingival pockets of periodontitis patients. Oral Microbiol Immunol 24:343–346
Lira AEG, Ramiro FS, Chiarelli FM, Dias RR, Feres M, Figueiredo LC et al (2013) Reduction in prevalence of archaea after periodontal therapy in subjects with generalized aggressive periodontitis. Aust Dent J 58:442–7
Bringuier A, Khelaifia S, Richet H, Aboudharam G, Drancourt M (2013) Real-time PCR quantification of Methanobrevibacter oralis in periodontitis. J Clin Microbiol 51:993–994
Huynh HTT, Pignoly M, Nkamga VD, Drancourt M, Aboudharam G. The repertoire of archaea cultivated from severe periodontitis. PloS One. 2015;10:e0121565.
Sogodogo E, Doumbo O, Aboudharam G, Kouriba B, Diawara O, Koita H et al (2019) First characterization of methanogens in oral cavity in Malian patients with oral cavity pathologies. BMC Oral Health 19:232
Faveri M, Gonçalves LFH, Feres M, Figueiredo LC, Gouveia LA, Shibli JA et al (2011) Prevalence and microbiological diversity of archaea in peri-implantitis subjects by 16S ribosomal RNA clonal analysis. J Periodontal Res 46:338–344
Aleksandrowicz P, Brzezińska-Błaszczyk E, Dudko A, Agier J (2020) Archaea occurrence in the subgingival biofilm in patients with peri-implantitis and periodontitis. Int J Periodontics Restorative Dent 40:677–683
Vianna ME, Conrads G, Gomes BPFA, Horz HP (2006) Identification and quantification of archaea involved in primary endodontic infections. J Clin Microbiol 44:1274–1282
Vickerman MM, Brossard KA, Funk DB, Jesionowski AM, Gill SR (2007) Phylogenetic analysis of bacterial and archaeal species in symptomatic and asymptomatic endodontic infections. J Med Microbiol 56:110–118
Jiang YT, Xia WW, Li CL, Jiang W, Liang JP (2009) Preliminary study of the presence and association of bacteria and archaea in teeth with apical periodontitis. Int Endod J 42:1096–1103
Efenberger M, Agier J, Pawłowska E, Brzezińska-Błaszczyk E (2015) Archaea prevalence in inflamed pulp tissues. Cent-Eur J Immunol 40:194–200
Brzezińska-Błaszczyk E, Pawłowska E, Płoszaj T, Witas H, Godzik U, Agier J (2018) Presence of archaea and selected bacteria in infected root canal systems. Can J Microbiol 64:317–326
Haines A, Metz G, Dilawari J, Blendis L, Wiggins H (1977) Breath-methane in patients with cancer of the large bowel. Lancet Lond Engl 2:481–483
Piqué JM, Pallarés M, Cusó E, Vilar-Bonet J, Gassull MA (1984) Methane production and colon cancer. Gastroenterology 87:601–605
Karlin DA, Jones RD, Stroehlein JR, Mastromarino AJ, Potter GD (1982) Breath methane excretion in patients with unresected colorectal cancer. J Natl Cancer Inst 69:573–576
Kashtan H, Rabau M, Peled Y, Milstein A, Wiznitzer T (1989) Methane production in patients with colorectal carcinoma. Isr J Med Sci 25:614–616
Segal I, Walker AR, Lord S, Cummings JH (1988) Breath methane and large bowel cancer risk in contrasting African populations. Gut 29:608–613
O’Keefe SJD, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J et al (2007) Why do African Americans get more colon cancer than Native Africans? J Nutr 137:175S-182S
Blais Lecours P, Marsolais D, Cormier Y, Berberi M, Haché C, Bourdages R, et al. Increased prevalence of Methanosphaera stadtmanae in inflammatory bowel diseases. PloS One. 2014;9:e87734.
Bjørneklett A, Fausa O, Midtvedt T (1983) Bacterial overgrowth in jejunal and ileal disease. Scand J Gastroenterol 18:289–298
McKay LF, Eastwood MA, Brydon WG (1985) Methane excretion in man—a study of breath, flatus, and faeces. Gut 26:69–74
Peled Y, Weinberg D, Hallak A, Gilat T (1987) Factors affecting methane production in humans Gastrointestinal diseases and alterations of colonic flora. Dig Dis Sci 32(267):71
Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y (2003) Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci 48:86–92
Scanlan PD, Shanahan F, Marchesi JR (2008) Human methanogen diversity and incidence in healthy and diseased colonic groups using mcrA gene analysis. BMC Microbiol 8:79
Ghavami SB, Rostami E, Sephay AA, Shahrokh S, Balaii H, Aghdaei HA et al (2018) Alterations of the human gut Methanobrevibacter smithii as a biomarker for inflammatory bowel diseases. Microb Pathog 117:285–289
Hwang L, Low K, Khoshini R, Melmed G, Sahakian A, Makhani M et al (2010) Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci 55:398–403
Pimentel M, Chow EJ, Lin HC (2003) Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome A double-blind randomized placebo controlled study. Am J Gastroenterol 98(412):9
Hubert S, Chadwick A, Wacher V, Coughlin O, Kokai-Kun J, Bristol A (2018) Development of a modified-release formulation of lovastatin targeted to intestinal methanogens implicated in irritable bowel syndrome with constipation. J Pharm Sci 107:662–671
Muskal SM, Sliman J, Kokai-Kun J, Pimentel M, Wacher V, Gottlieb K. Lovastatin lactone may improve irritable bowel syndrome with constipation (IBS-C) by inhibiting enzymes in the archaeal methanogenesis pathway. F1000Research. 2016;5:606.
Chatterjee S, Park S, Low K, Kong Y, Pimentel M (2007) The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol 102:837–841
Kim G, Deepinder F, Morales W, Hwang L, Weitsman S, Chang C et al (2012) Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Dig Dis Sci 57:3213–3218
Ghoshal U, Shukla R, Srivastava D, Ghoshal UC (2016) Irritable bowel syndrome, particularly the constipation-predominant form, involves an increase in Methanobrevibacter smithii, which is associated with higher methane production. Gut Liver 10:932–938
Bratten JR, Spanier J, Jones MP (2008) Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol 103:958–963
Rana SV, Sharma S, Sinha SK, Kaur H, Sikander A, Singh K (2009) Incidence of predominant methanogenic flora in irritable bowel syndrome patients and apparently healthy controls from North India. Dig Dis Sci 54:132–135
Chassard C, Dapoigny M, Scott KP, Crouzet L, Del’homme C, Marquet P et al (2012) Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther 35:828–38
Rajilić-Stojanović M, Biagi E, Heilig HGHJ, Kajander K, Kekkonen RA, Tims S et al (2011) Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 141:1792–1801
Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J et al (2017) Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 152:111-123.e8
Weaver GA, Krause JA, Miller TL, Wolin MJ (1986) Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut 27:698–704
Yazici C, Arslan DC, Abraham R, Cushing K, Keshavarzian A, Mutlu EA (2016) Breath methane levels are increased among patients with diverticulosis. Dig Dis Sci 61:2648–2654
Jang S-I, Kim J-H, Youn YH, Park H, Lee SI, Conklin JL (2010) Relationship between intestinal gas and the development of right colonic diverticula. J Neurogastroenterol Motil 16:418–423
Soares ACF, Lederman HM, Fagundes-Neto U, de Morais MB (2005) Breath methane associated with slow colonic transit time in children with chronic constipation. J Clin Gastroenterol 39:512–515
Pimentel M, Lin HC, Enayati P, van den Burg B, Lee H-R, Chen JH et al (2006) Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol 290:G1089-1095
Attaluri A, Jackson M, Valestin J, Rao SSC (2010) Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol 105:1407–1411
Kunkel D, Basseri RJ, Makhani MD, Chong K, Chang C, Pimentel M (2011) Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci 56:1612–1618
Fiedorek SC, Pumphrey CL, Casteel HB (1990) Breath methane production in children with constipation and encopresis. J Pediatr Gastroenterol Nutr 10:473–477
Feng X, Su Y, Jiang J, Li N, Ding W, Wang Z et al (2015) Changes in fecal and colonic mucosal microbiota of patients with refractory constipation after a subtotal colectomy. Am Surg 81:198–206
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al (2009) Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 106:2365–2370
Patil DP, Dhotre DP, Chavan SG, Sultan A, Jain DS, Lanjekar VB et al (2012) Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci 37:647–657
Mathur R, Amichai M, Chua KS, Mirocha J, Barlow GM, Pimentel M (2013) Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J Clin Endocrinol Metab 98:E698-702
Mbakwa CA, Penders J, Savelkoul PH, Thijs C, Dagnelie PC, Mommers M et al (2015) Gut colonization with Methanobrevibacter smithii is associated with childhood weight development. Obes Silver Spring Md 23:2508–2516
Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P et al (2005) Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes 2012(36):817–825
Million M, Angelakis E, Maraninchi M, Henry M, Giorgi R, Valero R et al (2005) Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes 2013(37):1460–1466
Million M, Thuny F, Angelakis E, Casalta J-P, Giorgi R, Habib G, et al. Lactobacillus reuteri and Escherichia coli in the human gut microbiota may predict weight gain associated with vancomycin treatment. Nutr Diabetes. 2013;3:e87.
Kayser BD, Prifti E, Lhomme M, Belda E, Dao M-C, Aron-Wisnewsky J et al (2019) Elevated serum ceramides are linked with obesity-associated gut dysbiosis and impaired glucose metabolism. Metabolomics Off J Metabolomic Soc 15:140
Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C et al (2010) Microbiota and SCFA in lean and overweight healthy subjects. Obes Silver Spring Md 18:190–195
Wilder-Smith CH, Olesen SS, Materna A, Drewes AM (2018) Breath methane concentrations and markers of obesity in patients with functional gastrointestinal disorders. United Eur Gastroenterol J 6:595–603
Belay N, Mukhopadhyay B, Conway de Macario E, Galask R, Daniels L (1990) Methanogenic bacteria in human vaginal samples. J Clin Microbiol 28:1666–8
Grine G, Drouet H, Fenollar F, Bretelle F, Raoult D, Drancourt M (2019) Detection of Methanobrevibacter smithii in vaginal samples collected from women diagnosed with bacterial vaginosis. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 38:1643–1649
Grine G, Lotte R, Chirio D, Chevalier A, Raoult D, Drancourt M et al (2019) Co-culture of Methanobrevibacter smithii with enterobacteria during urinary infection. EBioMedicine 43:333–337
Nkamga VD, Lotte R, Roger P-M, Drancourt M, Ruimy R (2016) Methanobrevibacter smithii and Bacteroides thetaiotaomicron cultivated from a chronic paravertebral muscle abscess. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 22:1008–1009
Drancourt M, Nkamga VD, Lakhe NA, Régis J-M, Dufour H, Fournier P-E et al (2017) Evidence of archaeal methanogens in brain abscess. Clin Infect Dis Off Publ Infect Dis Soc Am 65:1–5
Nkamga VD, Lotte R, Chirio D, Lonjon M, Roger P-M, Drancourt M et al (2018) Methanobrevibacter oralis detected along with Aggregatibacter actinomycetemcomitans in a series of community-acquired brain abscesses. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 24:207–208
Sogodogo E, Fellag M, Loukil A, Nkamga VD, Michel J, Dessi P et al (2019) Nine cases of methanogenic archaea in refractory sinusitis, an emerging clinical entity. Front Public Health 7:38
Drancourt M, Djemai K, Gouriet F, Grine G, Loukil A, Bedotto M, et al. Methanobrevibacter smithii archaemia in febrile patients with bacteremia, including those with endocarditis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;
Thauer RK. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson prize lecture. Microbiol Read Engl. 1998;144 ( Pt 9):2377–406.
Liu Y, Whitman WB (2008) Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci 1125:171–189
Deppenmeier U, Müller V, Gottschalk G (1996) Pathways of energy conservation in methanogenic archaea. Arch Microbiol 165:149–163
Blaut M (1994) Metabolism of methanogens. Antonie Van Leeuwenhoek 66:187–208
Thauer RK, Kaster A-K, Seedorf H, Buckel W, Hedderich R (2008) Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol 6:579–591
Muñoz-Tamayo R, Popova M, Tillier M, Morgavi DP, Morel J-P, Fonty G, et al. Hydrogenotrophic methanogens of the mammalian gut: functionally similar, thermodynamically different-a modelling approach. PloS One. 2019;14:e0226243.
Kurth JM, Op den Camp HJM, Welte CU. Several ways one goal-methanogenesis from unconventional substrates. Appl Microbiol Biotechnol. 2020;104:6839–54.
Daniels L, Fuchs G, Thauer RK, Zeikus JG (1977) Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol 132:118–126
O’Brien JM, Wolkin RH, Moench TT, Morgan JB, Zeikus JG (1984) Association of hydrogen metabolism with unitrophic or mixotrophic growth of Methanosarcina barkeri on carbon monoxide. J Bacteriol 158:373–375
Poehlein A, Schneider D, Soh M, Daniel R, Seedorf H (2018) Comparative genomic analysis of members of the genera Methanosphaera and Methanobrevibacter reveals distinct clades with specific potential metabolic functions. Archaea Vanc BC 2018:7609847
Ferry JG (2011) Fundamentals of methanogenic pathways that are key to the biomethanation of complex biomass. Curr Opin Biotechnol 22:351–357
Fricke WF, Seedorf H, Henne A, Krüer M, Liesegang H, Hedderich R et al (2006) The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2 for methane formation and ATP synthesis. J Bacteriol 188:642–658
Fu H, Metcalf WW (2015) Genetic basis for metabolism of methylated sulfur compounds in Methanosarcina species. J Bacteriol 197:1515–1524
Borrel G, McCann A, Deane J, Neto MC, Lynch DB, Brugère J-F et al (2017) Genomics and metagenomics of trimethylamine-utilizing archaea in the human gut microbiome. ISME J 11:2059–2074
Deppenmeier U (2002) The unique biochemistry of methanogenesis. Prog Nucleic Acid Res Mol Biol 71:223–283
van der Meijden P, Heythuysen HJ, Pouwels A, Houwen F, van der Drift C, Vogels GD (1983) Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch Microbiol 134:238–242
Taillefer M, Sparling R (2016) Glycolysis as the central core of fermentation. Adv Biochem Eng Biotechnol 156:55–77
Koch M, Dolfing J, Wuhrmann K, Zehnder AJ (1983) Pathways of propionate degradation by enriched methanogenic cultures. Appl Environ Microbiol 45:1411–1414
Macfarlane GT, Macfarlane S (2012) Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int 95:50–60
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340
Ndeh D, Rogowski A, Cartmell A, Luis AS, Baslé A, Gray J et al (2017) Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544:65–70
Schink B, Zeikus JG (1980) Microbial methanol formation: a major end product of pectin metabolism. Curr Microbiol 4:387–389
Neis EPJG, Dejong CHC, Rensen SS (2015) The role of microbial amino acid metabolism in host metabolism. Nutrients 7:2930–2946
Louis P, Flint HJ (2017) Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 19:29–41
Oliphant K, Allen-Vercoe E (2019) Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7:91
Potrykus J, White RL, Bearne SL (2008) Proteomic investigation of amino acid catabolism in the indigenous gut anaerobe Fusobacterium varium. Proteomics 8:2691–2703
Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5:e00889.
de Bok FA, Plugge CM, Stams AJ (2004) Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res 38:1368–1375
Dong X, Stams AJ (1995) Evidence for H2 and formate formation during syntrophic butyrate and propionate degradation. Anaerobe 1:35–39
Craciun S, Balskus EP (2012) Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A 109:21307–21312
Rath S, Heidrich B, Pieper DH, Vital M (2017) Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 5:54
Zhu Y, Jameson E, Crosatti M, Schäfer H, Rajakumar K, Bugg TDH et al (2014) Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A 111:4268–4273
Creighbaum AJ, Ticak T, Shinde S, Wang X, Ferguson DJ (2019) Examination of the glycine betaine-dependent methylotrophic methanogenesis pathway: insights into anaerobic quaternary amine methylotrophy. Front Microbiol 10:2572
Doi Y (2019) Glycerol metabolism and its regulation in lactic acid bacteria. Appl Microbiol Biotechnol 103:5079–5093
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52:289–297
Jeyanathan J, Martin C, Morgavi DP (2014) The use of direct-fed microbials for mitigation of ruminant methane emissions: a review. Animal 8:250–261
Belenguer A, Duncan SH, Holtrop G, Anderson SE, Lobley GE, Flint HJ (2007) Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl Environ Microbiol 73:6526–6533
Scheifinger CC, Linehan B, Wolin MJ (1975) H2 production by Selenomonas ruminantium in the absence and presence of methanogenic bacteria. Appl Microbiol 29:480–483
Batstone DJ, Picioreanu C, van Loosdrecht MCM (2006) Multidimensional modelling to investigate interspecies hydrogen transfer in anaerobic biofilms. Water Res 40:3099–3108
Müller N, Worm P, Schink B, Stams AJM, Plugge CM (2010) Syntrophic butyrate and propionate oxidation processes: from genomes to reaction mechanisms. Environ Microbiol Rep 2:489–499
McInerney MJ, Sieber JR, Gunsalus RP (2009) Syntrophy in anaerobic global carbon cycles. Curr Opin Biotechnol 20:623–632
Stams AJM, Plugge CM (2009) Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol 7:568–577
Hansen EE, Lozupone CA, Rey FE, Wu M, Guruge JL, Narra A et al (2011) Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc Natl Acad Sci U S A 108(Suppl 1):4599–4606
Ren N, Xing D, Rittmann BE, Zhao L, Xie T, Zhao X (2007) Microbial community structure of ethanol type fermentation in bio-hydrogen production. Environ Microbiol 9:1112–1125
Carbonero F, Benefiel AC, Gaskins HR (2012) Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol 9:504–518
Tsuji K, Yagi T (1980) Significance of hydrogen burst from growing cultures of Desulfovibrio vulgaris, Miyazaki, and the role of hydrogenase and cytochrome c3 in energy production system. Arch Microbiol 125:35–42
Traore AS, Hatchikian CE, Belaich JP, Le Gall J (1981) Microcalorimetric studies of the growth of sulfate-reducing bacteria: energetics of Desulfovibrio vulgaris growth. J Bacteriol 145:191–199
Bryant MP, Campbell LL, Reddy CA, Crabill MR (1977) Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol 33:1162–1169
Gibson GR (1990) Physiology and ecology of the sulphate-reducing bacteria. J Appl Bacteriol 69:769–797
Hoh CY, Cord-Ruwisch R (1996) A practical kinetic model that considers endproduct inhibition in anaerobic digestion processes by including the equilibrium constant. Biotechnol Bioeng 51:597–604
Levy R, Borenstein E (2013) Metabolic modeling of species interaction in the human microbiome elucidates community-level assembly rules. Proc Natl Acad Sci U S A 110:12804–12809
Shoaie S, Karlsson F, Mardinoglu A, Nookaew I, Bordel S, Nielsen J (2013) Understanding the interactions between bacteria in the human gut through metabolic modeling. Sci Rep 3:2532
Stolyar S, Van Dien S, Hillesland KL, Pinel N, Lie TJ, Leigh JA et al (2007) Metabolic modeling of a mutualistic microbial community. Mol Syst Biol 3:92
Smith NW, Shorten PR, Altermann E, Roy NC, McNabb WC (2020) Competition for hydrogen prevents coexistence of human gastrointestinal hydrogenotrophs in continuous culture. Front Microbiol 11:1073
Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T et al (2014) The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206-214
Huynh HTT, Pignoly M, Drancourt M, Aboudharam G (2017) A new methanogen “Methanobrevibacter massiliense” isolated in a case of severe periodontitis. BMC Res Notes 10:657
Horz HP, Robertz N, Vianna ME, Henne K, Conrads G (2015) Relationship between methanogenic archaea and subgingival microbial complexes in human periodontitis. Anaerobe 35:10–12
Horz H-P, Conrads G. Methanogenic archaea and oral infections - ways to unravel the black box. J Oral Microbiol. 2011;3.
Faveri M, Figueiredo LC, Duarte PM, Mestnik MJ, Mayer MPA, Feres M (2009) Microbiological profile of untreated subjects with localized aggressive periodontitis. J Clin Periodontol 36:739–749
Mansfield JM, Campbell JH, Bhandari AR, Jesionowski AM, Vickerman MM (2012) Molecular analysis of 16S rRNA genes identifies potentially periodontal pathogenic bacteria and archaea in the plaque of partially erupted third molars. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg 70:1507-1514.e1–6
Miller TL, Weaver GA, Wolin MJ (1984) Methanogens and anaerobes in a colon segment isolated from the normal fecal stream. Appl Environ Microbiol 48:449–450
Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J (2016) Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65:57–62
Traore SI, Khelaifia S, Armstrong N, Lagier JC, Raoult D (2019) Isolation and culture of Methanobrevibacter smithii by co-culture with hydrogen-producing bacteria on agar plates. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 25:1561.e1-1561.e5
Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PloS One. 2013;8:e66019.
Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R et al (2014) Human genetics shape the gut microbiome. Cell 159:789–799
Vanderhaeghen S, Lacroix C, Schwab C. Methanogen communities in stools of humans of different age and health status and co-occurrence with bacteria. FEMS Microbiol Lett. 2015;362:fnv092.
Teigen L, Mathai PP, Matson M, Lopez S, Kozysa D, Kabage AJ, et al. Methanogen abundance thresholds capable of differentiating in vitro methane production in human stool samples. Dig Dis Sci. 2020;
Lyu Z. Back to the source: molecular identification of methanogenic archaea as markers of colonic methane production. Dig Dis Sci. 2021;
Macy JM, Probst I (1979) The biology of gastrointestinal bacteroides. Annu Rev Microbiol 33:561–594
Shah HN, Gharbia SE (1993) Ecophysiology and taxonomy of Bacteroides and related taxa. Clin Infect Dis Off Publ Infect Dis Soc Am 16(Suppl 4):S160-167
Ito T, Gallegos R, Matano LM, Butler NL, Hantman N, Kaili M, et al. Genetic and biochemical analysis of anaerobic respiration in Bacteroides fragilis and its importance in vivo. mBio. 2020;11.
Takahashi N (2015) Oral microbiome metabolism: from “who are they?” to “what are they doing?” J Dent Res 94:1628–1637
Takahashi N, Yamada T (2000) Glucose metabolism by Prevotella intermedia and Prevotella nigrescens. Oral Microbiol Immunol 15:188–195
Bryant MP, Small N, Bouma C, Robinson IM (1958) Characteristics of ruminal anaerobic celluloytic cocci and Cillobacterium cellulosolvens n sp. J Bacteriol 76(529):37
Fu X, Liu Z, Zhu C, Mou H, Kong Q (2019) Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit Rev Food Sci Nutr 59:S130–S152
Delwiche EA, Pestka JJ, Tortorello ML (1985) The veillonellae: gram-negative cocci with a unique physiology. Annu Rev Microbiol 39:175–193
Ng SK, Hamilton IR (1973) Carbon dioxide fixation by Veillonella parvula M 4 and its relation to propionic acid formation. Can J Microbiol 19:715–723
Yoshida A, Nishimura T, Kawaguchi H, Inui M, Yukawa H (2005) Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl Environ Microbiol 71:6762–6768
Orencio-Trejo M, Utrilla J, Fernández-Sandoval MT, Huerta-Beristain G, Gosset G, Martinez A (2010) Engineering the Escherichia coli fermentative metabolism. Adv Biochem Eng Biotechnol 121:71–107
Converti A, Perego P (2002) Use of carbon and energy balances in the study of the anaerobic metabolism of Enterobacter aerogenes at variable starting glucose concentrations. Appl Microbiol Biotechnol 59:303–309
Maru BT, López F, Kengen SWM, Constantí M, Medina F (2016) Dark fermentative hydrogen and ethanol production from biodiesel waste glycerol using a co-culture of Escherichia coli and Enterobacter sp. Fuel 186:375–384
Kurokawa T, Tanisho S (2005) Effects of formate on fermentative hydrogen production by Enterobacter aerogenes. Mar Biotechnol N Y N 7:112–118
Zeng AP, Biebl H, Schlieker H, Deckwer WD (1993) Pathway analysis of glycerol fermentation by Klebsiella pneumoniae: regulation of reducing equivalent balance and product formation. Enzyme Microb Technol 15:770–779
Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY (2012) Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 42:1597–1608
Ochuba GU, von Riesen VL (1980) Fermentation of polysaccharides by Klebsielleae and other facultative bacilli. Appl Environ Microbiol 39:988–992
Morotomi M, Nagai F, Watanabe Y (2012) Description of Christensenella minuta gen nov sp nov isolated from human faeces which forms a distinct branch in the order Clostridiales and proposal of Christensenellaceae fam nov. Int J Syst Evol Microbiol 62(144):9
Bender KS, Yen H-C, Wall JD (2006) Analysing the metabolic capabilities of Desulfovibrio species through genetic manipulation. Biotechnol Genet Eng Rev 23:157–174
Baffert C, Kpebe A, Avilan L, Brugna M (2019) Hydrogenases and H2 metabolism in sulfate-reducing bacteria of the Desulfovibrio genus. Adv Microb Physiol 74:143–189
Hansen TA (1994) Metabolism of sulfate-reducing prokaryotes. Antonie Van Leeuwenhoek 66:165–185
Janda JM, Abbott SL, Khashe S, Robin T (1996) Biochemical investigations of biogroups and subspecies of Morganella morganii. J Clin Microbiol 34:108–113
Janda JM, Abbott SL. Morganella. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–7. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm01155
Penner JL. Proteus. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–17. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm01162
Downes J, Vartoukian SR, Dewhirst FE, Izard J, Chen T, Yu WH et al (2009) Pyramidobacter piscolens gen nov sp nov a member of the phylum Synergistetes isolated from the human oral cavity. Int J Syst Evol Microbiol 59(972):80
Frederiksen W. Citrobacter. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–23. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm01143
Homann T, Tag C, Biebl H, Deckwer W-D, Schink B (1990) Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains. Appl Microbiol Biotechnol 33:121–126
Collier DN, Hager PW, Phibbs PV (1996) Catabolite repression control in the pseudomonads. Res Microbiol 147:551–561
Babaei P, Ghasemi-Kahrizsangi T, Marashi S-A. Modeling the differences in biochemical capabilities of pseudomonas species by flux balance analysis: how good are genome-scale metabolic networks at predicting the differences? ScientificWorldJournal. 2014;2014:416289.
Lin Y-C, Cornell WC, Jo J, Price-Whelan A, Dietrich LEP. The Pseudomonas aeruginosa complement of lactate dehydrogenases enables use of d- and l-lactate and metabolic cross-feeding. mBio. 2018;9.
Loesche WJ, Bretz WA, Kerschensteiner D, Stoll J, Socransky SS, Hujoel P et al (1990) Development of a diagnostic test for anaerobic periodontal infections based on plaque hydrolysis of benzoyl-DL-arginine-naphthylamide. J Clin Microbiol 28:1551–1559
Norris SJ, Paster BJ, Smibert RM. Treponema. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–42. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm01249
Singh RP (2019) Glycan utilisation system in Bacteroides and Bifidobacteria and their roles in gut stability and health. Appl Microbiol Biotechnol 103:7287–7315
Salyers AA, West SE, Vercellotti JR, Wilkins TD (1977) Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol 34:529–533
Hill GB, Ayers OM, Kohan AP (1987) Characteristics and sites of infection of Eubacterium nodatum, Eubacterium timidum, Eubacterium brachy, and other asaccharolytic eubacteria. J Clin Microbiol 25:1540–1545
Derrien M, Vaughan EE, Plugge CM, de Vos WM (2004) Akkermansia muciniphila gen nov sp nov a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54(1469):76
Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms. 2018;6.
Schaal KP, Yassin AA. Actinomyces. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–112. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm00012
Distler W, Kröncke A (1981) Acid formation by mixed cultures of dental plaque bacteria Actinomyces and Veillonella. Arch Oral Biol 26:123–126
Sivakanesan R, Dawes EA (1980) Anaerobic glucose and serine metabolism in Staphylococcus epidermidis. J Gen Microbiol 118:143–157
Strasters KC, Winkler KC (1963) Carbohydrate metabolism of Staphylococcus aureus. J Gen Microbiol 33:213–229
Rosenstein R, Götz F (2000) Staphylococcal lipases: biochemical and molecular characterization. Biochimie 82:1005–1014
Neijssel OM, Snoep JL, Teixeira de Mattos MJ (1997) Regulation of energy source metabolism in streptococci. Soc Appl Bacteriol Symp Ser 26:12S-19S
Willenborg J, Goethe R (2016) Metabolic traits of pathogenic streptococci. FEBS Lett 590:3905–3919
Mendz GL, Ball GE, Meek DJ (1997) Pyruvate metabolism in Campylobacter spp. Biochim Biophys Acta 1334:291–302
Vandamme P, Dewhirst FE, Paster BJ, On SLW. Campylobacter. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–27. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm01071
McCubbin T, Gonzalez-Garcia RA, Palfreyman RW, Stowers C, Nielsen LK, Marcellin E. A pan-genome guided metabolic network reconstruction of five propionibacterium species reveals extensive metabolic diversity. Genes. 2020;11.
Patrick S, McDowell A. Propionibacterium. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–29. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm00167
Stewart CS, Duncan SH, Cave DR (2004) Oxalobacter formigenes and its role in oxalate metabolism in the human gut. FEMS Microbiol Lett 230:1–7
Kageyama A, Benno Y (2000) Catenibacterium mitsuokai gen nov sp nov a gram-positive anaerobic bacterium isolated from human faeces. Int J Syst Evol Microbiol 50(Pt 4):1595–9
Patel BKC. Papillibacter. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–4. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm00677
Sakamoto M, Benno Y (2006) Reclassification of Bacteroides distasonis Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasonis gen nov comb nov Parabacteroides goldsteinii comb nov and Parabacteroides merdae comb nov. Int J Syst Evol Microbiol 56(1599):605
Ezaki T, Kawamura Y, Li N, Li ZY, Zhao L, Shu S (2001) Proposal of the genera Anaerococcus gen nov Peptoniphilus gen nov and Gallicola gen nov for members of the genus Peptostreptococcus. Int J Syst Evol Microbiol 51(1521):8
Grech‐Mora I, Fardeau M-L, Garcia J-L, Ollivier B. Sporobacter. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–6. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm00679
Sakamoto M, Ikeyama N, Toyoda A, Murakami T, Mori H, Iino T et al (2020) Dialister hominis sp nov isolated from human faeces. Int J Syst Evol Microbiol 70(589):95
Posch G, Sekot G, Friedrich V, Megson ZA, Koerdt A, Messner P et al (2012) Glycobiology aspects of the periodontal pathogen Tannerella forsythia. Biomolecules 2:467–482
Sakamoto M, Tanner ACR, Benno Y. Tannerella. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 3]. p. 1–9. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm00248
Gophna U, Konikoff T, Nielsen HB (2017) Oscillospira and related bacteria - from metagenomic species to metabolic features. Environ Microbiol 19:835–841
Drancourt M, Bollet C, Carta A, Rousselier P (2001) Phylogenetic analyses of Klebsiella species delineate Klebsiella and Raoultella gen nov with description of Raoultella ornithinolytica comb nov Raoultella terrigena comb nov and Raoultella planticola comb nov. Int J Syst Evol Microbiol 51(925):32
Kim T, Cho S, Woo HM, Lee S-M, Lee J, Um Y et al (2017) High production of 2,3-butanediol from glycerol without 1,3-propanediol formation by Raoultella ornithinolytica B6. Appl Microbiol Biotechnol 101:2821–2830
Estrela AB, Abraham WR (2010) Brevundimonas vancanneytii sp nov isolated from blood of a patient with endocarditis. Int J Syst Evol Microbiol 60:2129–34
Rainey FA. Allisonella. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 4]. p. 1–3. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm00688
Arthur LO, Bulla LA, Julian GS, Nakamura LK (1973) Carbohydrate metabolism in Agrobacterium tumefaciens. J Bacteriol 116:304–313
Brown SA, Whiteley M. Characterization of the l-lactate dehydrogenase from Aggregatibacter actinomycetemcomitans. PloS One. 2009;4:e7864.
Godon J-J, Morinière J, Moletta M, Gaillac M, Bru V, Delgènes J-P (2005) Rarity associated with specific ecological niches in the bacterial world: the “Synergistes” example. Environ Microbiol 7:213–224
Sly LI, Wen A, Fegan M. Delftia. Bergeys Man Syst Archaea Bact [Internet]. American Cancer Society; 2015 [cited 2021 Mar 4]. p. 1–7. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781118960608.gbm00946
Laffineur K, Avesani V, Cornu G, Charlier J, Janssens M, Wauters G et al (2003) Bacteremia due to a novel Microbacterium species in a patient with leukemia and description of Microbacterium paraoxydans sp nov. J Clin Microbiol 41(2242):6
Conway de Macario E, Macario AJL (2009) Methanogenic archaea in health and disease: a novel paradigm of microbial pathogenesis. Int J Med Microbiol IJMM 299:99–108
Brugère J-F, Borrel G, Gaci N, Tottey W, O’Toole PW, Malpuech-Brugère C (2014) Archaebiotics: proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes 5:5–10
Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F et al (2018) Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol 38:2225–2235
Tidjani Alou M, Lagier J-C, Raoult D (2016) Diet influence on the gut microbiota and dysbiosis related to nutritional disorders. Human Microbiome Journal 1:3–11
Cheng YF, Jin W, Mao SY, Zhu W-Y (2013) Production of citrate by anaerobic fungi in the presence of co-culture methanogens as revealed by 1H NMR spectrometry. Asian-Australas J Anim Sci 26:1416–1423
Lewis WH, Sendra KM, Embley TM, Esteban GF. Morphology and phylogeny of a new species of anaerobic ciliate, Trimyema finlayi n. sp., with endosymbiotic methanogens. Front Microbiol. 2018;9:140.
Funding
This study was supported by the Mediterranée Infection Foundation as well as the Agence Nationale de la Recherche under the program “Investissements d’avenir” under reference Mediterranée Infection 10-IAIHU-03.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Djemai, K., Drancourt, M. & Tidjani Alou, M. Bacteria and Methanogens in the Human Microbiome: a Review of Syntrophic Interactions. Microb Ecol 83, 536–554 (2022). https://doi.org/10.1007/s00248-021-01796-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01796-7