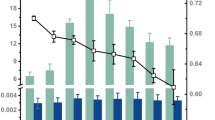
Abstract
Large-scale open microalgae cultivation has tremendous potential to make a significant contribution to replacing petroleum-based fuels with biofuels. Open algal cultures are unavoidably inhabited with a diversity of microbes that live on, influence, and shape the fate of these ecosystems. However, there is little understanding of the resilience and stability of the microbial communities in engineered semicontinuous algal systems. To evaluate the dynamics and resilience of the microbial communities in microalgae biofuel cultures, we conducted a longitudinal study on open systems to compare the temporal profiles of the microbiota from two multigenerational algal cohorts, which include one seeded with the microbiota from an in-house culture and the other exogenously seeded with a natural-occurring consortia of bacterial species harvested from the Pacific Ocean. From these month-long, semicontinuous open microalga Nannochloropsis salina cultures, we sequenced a time-series of 46 samples, yielding 8804 operational taxonomic units derived from 9,160,076 high-quality partial 16S rRNA sequences. We provide quantitative evidence that clearly illustrates the development of microbial community is associated with microbiota ancestry. In addition, N. salina growth phases were linked with distinct changes in microbial phylotypes. Alteromonadeles dominated the community in the N. salina exponential phase whereas Alphaproteobacteria and Flavobacteriia were more prevalent in the stationary phase. We also demonstrate that the N. salina-associated microbial community in open cultures is diverse, resilient, and dynamic in response to environmental perturbations. This knowledge has general implications for developing and testing design principles of cultivated algal systems.






Similar content being viewed by others
References
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286. doi:10.1016/j.copbio.2010.03.005
Georgianna DR, Mayfield SP (2012) Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 488:329–335. doi:10.1038/nature11479
Carney LT, Reinsch SS, Lane PD, Solberg OD, Jansen LS, Williams KP, Trent JD, Lane TW (2014) Microbiome analysis of a microalgal mass culture growing in municipal wastewater in a prototype OMEGA photobioreactor. Algal Res. doi:10.1016/j.algal.2013.11.006
Kazamia E, Aldridge DC, Smith AG (2012) Synthetic ecology—a way forward for sustainable algal biofuel production? J Biotechnol 162:163–169. doi:10.1016/j.jbiotec.2012.03.022
Kayser H (1979) Growth interactions between marine dinoflagellates in multispecies culture experiments. Mar Biol 52:357–369. doi:10.1007/bf00389077
Lee S-O, Kato J, Takiguchi N, Kuroda A, Ikeda T, Mitsutani A, Ohtake H (2000) Involvement of an extracellular protease in algicidal activity of the marine bacterium Pseudoalteromonas sp. strain A28. Appl Environ Microbiol 66:4334–4339. doi:10.1128/aem.66.10.4334-4339.2000
Geng H, Belas R (2010) Molecular mechanisms underlying roseobacter-phytoplankton symbioses. Curr Opin Biotechnol 21:332–338. doi:10.1016/j.copbio.2010.03.013
McCann KS (2000) The diversity-stability debate. Nature 405:228–233. doi:10.1038/35012234
Huber JA, Welch DBM, Morrison HG, Huse SM, Neal PR, Butterfield DA, Sogin ML (2007) Microbial population structures in the deep marine biosphere. Science 318:97–100. doi:10.1126/science.1146689
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, De Vos P, Verstraete W, Boon N (2009) Initial community evenness favours functionality under selective stress. Nature 458:623–626. doi:10.1038/nature07840
Wilsey BJ, Polley HW (2002) Reductions in grassland species evenness increase dicot seedling invasion and spittle bug infestation. Ecol Lett 5:676–684. doi:10.1046/j.1461-0248.2002.00372.x
Bell T, Newman JA, Silverman BW, Turner SL, Lilley AK (2005) The contribution of species richness and composition to bacterial services. Nature 436:1157–1160. doi:10.1038/nature03891
Werner JJ, Knights D, Garcia ML, Scalfone NB, Smith S, Yarasheski K, Cummings TA, Beers AR, Knight R, Angenent LT (2011) Bacterial community structures are unique and resilient in full-scale bioenergy systems. Proc Natl Acad Sci U S A 108:4158–4163. doi:10.1073/pnas.1015676108
Krohn-Molt I, Wemheuer B, Alawi M, Poehlein A, Güllert S, Schmeisser C, Pommerening-Röser A, Grundhoff A, Daniel R, Hanelt D, Streit WR (2013) Metagenome survey of a multispecies and alga-associated biofilm revealed key elements of bacterial-algal interactions in photobioreactors. Appl Environ Microbiol 79:6196–6206. doi:10.1128/aem.01641-13
Lakaniemi A-M, Hulatt CJ, Wakeman KD, Thomas DN, Puhakka JA (2012) Eukaryotic and prokaryotic microbial communities during microalgal biomass production. Bioresour Technol 124:387–393. doi:10.1016/j.biortech.2012.08.048
Harrison P, Waters R, Taylor F (1980) A broad spectrum artificial sea water medium for coastal and open ocean phytoplankton. J Phycol 16:28–35. doi:10.1111/j.0022-3646.1980.00028.x
Wood ED, Armstrong FAJ, Richards FA (1967) Determination of nitrate in sea water by cadmium-copper reduction to nitrite. J Mar Biol Assoc UK 47:23–31. doi:10.1017/S002531540003352X
Dick WA, Tabatabai MA (1977) Determination of orthophosphate in aqueous solutions containing labile organic and inorganic phosphorus compounds. J Environ Qual 6:82–85. doi:10.2134/jeq1977.00472425000600010018x
Bartram AK, Lynch MDJ, Stearns JC, Moreno-Hagelsieb G, Neufeld JD (2011) Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Appl Environ Microbiol 77:3846–3852. doi:10.1128/aem.02772-10
Rodrigue S, Materna AC, Timberlake SC, Blackburn MC, Malmstrom RR, Alm EJ, Chisholm SW (2010) Unlocking short read sequencing for metagenomics. PLoS One 5:e11840. doi:10.1371/journal.pone.0011840
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi:10.1038/nmeth.f.303
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi:10.1128/aem.03006-05
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270. doi:10.2307/4615964
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: an effective distance metric for microbial community comparison. ISME J 5:169–172. doi:10.1038/ismej.2010.133
Wood AM, Everroad RC, Wingard LM (2005) Measuring growth rates in microalgal cultures. In: Anderson RA (ed) Algal culturing techniques. Elsevier Academic Press, Burlington, pp 269–286
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi:10.1128/AEM.71.12.8228-8235.2005
González JM, Simó R, Massana R, Covert JS, Casamayor EO, Pedrós-Alió C, Moran MA (2000) Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl Environ Microbiol 66:4237–4246
Geng H, Belas R (2010) Molecular mechanisms underlying roseobacter–phytoplankton symbioses. Curr Opin Biotechnol 21:332–338. doi:10.1016/j.copbio.2010.03.013
Raes J, Bork P (2008) Molecular eco-systems biology: towards an understanding of community function. Nat Rev Microbiol 6:693–699. doi:10.1038/nrmicro1935
Fuhrman JA (2009) Microbial community structure and its functional implications. Nature 459:193–199. doi:10.1038/nature08058
Pedros-Alio C (2006) Marine microbial diversity: can it be determined? Trends Microbiol 14:257–263. doi:10.1016/j.tim.2006.04.007
Tada Y, Taniguchi A, Nagao I, Miki T, Uematsu M, Tsuda A, Hamasaki K (2011) Differing growth responses of major phylogenetic groups of marine bacteria to natural phytoplankton blooms in the western North Pacific Ocean. Appl Environ Microbiol 77:4055–4065. doi:10.1128/AEM.02952-10
McCarren J, Becker JW, Repeta DJ, Shi Y, Young CR, Malmstrom RR, Chisholm SW, DeLong EF (2010) Microbial community transcriptomes reveal microbes and metabolic pathways associated with dissolved organic matter turnover in the sea. Proc Natl Acad Sci U S A 107:16420–16427. doi:10.1073/pnas.1010732107
Gomez-Pereira PR, Schuler M, Fuchs BM, Bennke C, Teeling H, Waldmann J, Richter M, Barbe V, Bataille E, Glockner FO, Amann R (2012) Genomic content of uncultured Bacteroidetes from contrasting oceanic provinces in the North Atlantic Ocean. Environ Microbiol 14:52–66. doi:10.1111/j.1462-2920.2011.02555.x
Edwards JL, Smith DL, Connolly J, McDonald JE, Cox MJ, Joint I, Edwards C, McCarthy AJ (2010) Identification of carbohydrate metabolism genes in the metagenome of a marine biofilm community shown to be dominated by Gammaproteobacteria and Bacteroidetes. Genes 1:371–384
Teeling H, Fuchs BM, Becher D, Klockow C, Gardebrecht A, Bennke CM, Kassabgy M, Huang S, Mann AJ, Waldmann J, Weber M, Klindworth A, Otto A, Lange J, Bernhardt J, Reinsch C, Hecker M, Peplies J, Bockelmann FD, Callies U, Gerdts G, Wichels A, Wiltshire KH, Glockner FO, Schweder T, Amann R (2012) Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 336:608–611. doi:10.1126/science.1218344
Geng H, Bruhn JB, Nielsen KF, Gram L, Belas R (2008) Genetic dissection of tropodithietic acid biosynthesis by marine roseobacters. Appl Environ Microbiol 74:1535–1545. doi:10.1128/AEM.02339-07
Alavi M, Miller T, Erlandson K, Schneider R, Belas R (2001) Bacterial community associated with Pfiesteria-like dinoflagellate cultures. Environ Microbiol 3:380–396
Wagner-Dobler I, Biebl H (2006) Environmental biology of the marine Roseobacter lineage. Annu Rev Microbiol 60:255–280. doi:10.1146/annurev.micro.60.080805.142115
Rinta-Kanto JM, Sun S, Sharma S, Kiene RP, Moran MA (2012) Bacterial community transcription patterns during a marine phytoplankton bloom. Environ Microbiol 14:228–239. doi:10.1111/j.1462-2920.2011.02602.x
Buchan A, Gonzalez JM, Moran MA (2005) Overview of the marine Roseobacter lineage. Appl Environ Microbiol 71:5665–5677. doi:10.1128/AEM.71.10.5665-5677.2005
Cude WN, Mooney J, Tavanaei AA, Hadden MK, Frank AM, Gulvik CA, May AL, Buchan A (2012) Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp. strain Y4I. Appl Environ Microbiol 78:4771–4780. doi:10.1128/aem.00297-12
Brinkhoff T, Bach G, Heidorn T, Liang L, Schlingloff A, Simon M (2004) Antibiotic production by a Roseobacter clade-affiliated species from the German Wadden Sea and its antagonistic effects on indigenous isolates. Appl Environ Microbiol 70:2560–2565
Geng H, Belas R (2010) Expression of tropodithietic acid biosynthesis is controlled by a novel autoinducer. J Bacteriol 192:4377–4387. doi:10.1128/JB.00410-10
Fuhrman JA, Hewson I, Schwalbach MS, Steele JA, Brown MV, Naeem S (2006) Annually reoccurring bacterial communities are predictable from ocean conditions. Proc Natl Acad Sci U S A 103:13104–13109. doi:10.1073/pnas.0602399103
Krohn-Molt I, Wemheuer B, Alawi M, Poehlein A, Gullert S, Schmeisser C, Pommerening-Roser A, Grundhoff A, Daniel R, Hanelt D, Streit WR (2013) Metagenome survey of a multispecies and alga-associated biofilm revealed key elements of bacterial-algal interactions in photobioreactors. Appl Environ Microbiol 79:6196–6206. doi:10.1128/AEM.01641-13
Peng Y, Leung HC, Yiu SM, Chin FY (2011) Meta-IDBA: a de Novo assembler for metagenomic data. Bioinformatics 27:i94–101. doi:10.1093/bioinformatics/btr216
Grossart HP, Levold F, Allgaier M, Simon M, Brinkhoff T (2005) Marine diatom species harbour distinct bacterial communities. Environ Microbiol 7:860–873. doi:10.1111/j.1462-2920.2005.00759.x
Paul C, Pohnert G (2011) Interactions of the algicidal bacterium Kordia algicida with diatoms: regulated protease excretion for specific algal lysis. PLoS One 6:e21032. doi:10.1371/journal.pone.0021032
Lovejoy C, Bowman JP, Hallegraeff GM (1998) Algicidal effects of a novel marine pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl Environ Microbiol 64:2806–2813
Mayali X, Azam F (2004) Algicidal bacteria in the sea and their impact on algal blooms1. J Eukaryot Microbiol 51:139–144. doi:10.1111/j.1550-7408.2004.tb00538.x
Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438: 90–93. http://www.nature.com/nature/journal/v438/n7064/suppinfo/nature04056_S1.html
Amin SA, Green DH, Hart MC, Kupper FC, Sunda WG, Carrano CJ (2009) Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc Natl Acad Sci U S A 106:17071–17076. doi:10.1073/pnas.0905512106
Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI (2013) The long-term stability of the human gut microbiota. Science 341. doi:10.1126/science.1237439
Rho M, Tang H, Ye Y (2010) FragGeneScan: predicting genes in short and error-prone reads. Nucleic Acids Res 38:e191. doi:10.1093/nar/gkq747
Hashsham SA, Fernandez AS, Dollhopf SL, Dazzo FB, Hickey RF, Tiedje JM, Criddle CS (2000) Parallel processing of substrate correlates with greater functional stability in methanogenic bioreactor communities perturbed by glucose. Appl Environ Microbiol 66:4050–4057
Acknowledgments
This work was supported by the Laboratory Directed Research and Development Program at Sandia National Laboratories, which is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the US Department of Energy’s National Nuclear Security Administration under Contract DE-AC04-94AL85000. Additional funding was provided by the US Department of Energy (DOE) Genomic Science Program under contract SCW1039.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Rarefaction analysis comparing microbial community of species richness (Chao1) and diversity (Shannon index) in 16S libraries from algal microbiota. Error bars depicted standard deviations during iterative resamplings from each duplicated samples. (JPG 2481 kb)
Fig. S2
A PCA plot based on unweighted Unifrac distance generally, with few exceptions, separates two groups of microbial community. Total ammonia-disturbed samples in passage 2 day 4 were excluded in this case to allow environmental conditions in those samples to be comparable. The origins of samples were indicated by colors (see legend). (JPG 73 kb)
Fig. S3
The comparison of algal growth rate as a function of species richness (a) and as a function of population diversity (b). Algal growth rate was significantly associated with microbial community diversity (P < 0.05). (JPG 1049 kb)
Rights and permissions
About this article
Cite this article
Geng, H., Sale, K.L., Tran-Gyamfi, M.B. et al. Longitudinal Analysis of Microbiota in Microalga Nannochloropsis salina Cultures. Microb Ecol 72, 14–24 (2016). https://doi.org/10.1007/s00248-016-0746-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0746-4