Abstract
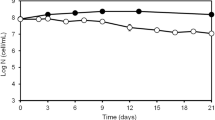
Vibrio fischeri is a bioluminescent bacterial symbiont of sepiolid squids (Cephalopoda: Sepiolidae) and monocentrid fishes (Actinopterygii: Monocentridae). V. fischeri exhibit competitive dominance within the allopatrically distributed squid genus Euprymna, which have led to the evolution of V. fischeri host specialists. In contrast, the host genus Sepiola contains sympatric species that is thought to have given rise to V. fischeri that have evolved as host generalists. Given that these ecological lifestyles may have a direct effect upon the growth spectrum and survival limits in contrasting environments, optimal growth ranges were obtained for numerous V. fischeri isolates from both free-living and host environments. Upper and lower limits of growth were observed in sodium chloride concentrations ranging from 0.0% to 9.0%. Sepiola symbiotic isolates possessed the least variation in growth throughout the entire salinity gradient, whereas isolates from Euprymna were the least uniform at <2.0% NaCl. V. fischeri fish symbionts (CG101 and MJ101) and all free-living strains were the most dissimilar at >5.0% NaCl. Growth kinetics of symbiotic V. fischeri strains were also measured under a range of salinity and temperature combinations. Symbiotic V. fischeri ES114 and ET101 exhibited a synergistic effect for salinity and temperature, where significant differences in growth rates due to salinity existed only at low temperatures. Thus, abiotic factors such as temperature and salinity have differential effects between free-living and symbiotic strains of V. fischeri, which may alter colonization efficiency prior to infection.






Similar content being viewed by others
References
Farmer J III, Janda JM (2001) Family Vibrionaceae. In: Brenner D, Krieg NR, Staley JT (eds) Bergy’s manual of systematic bacteriology, vol 2. Springer, New York, NY, pp 491–555
Urakawa H, Rivera ING (2006) Aquatic Environment. In: Thompson FL, Austin B, Swings J (eds) The biology of Vibrios. ASM, Washington, D.C., pp 175–189
McDougald D, Kjelleberg S (2006) Adaptive responses to Vibrios. In: Thompson FL, Austin B, Swings J (eds) The biology of Vibrios. ASM, Washington, D.C., pp 133–155
Bartlett DH (2006) Extremophilic Vibrionaceae. In: Thompson FL, Austin B, Swings J (eds) The biology of Vibrios. ASM, Washington, D.C., pp 156–171
Nishiguchi MK, Nair VA (2003) Evolution of pathogenicity and symbioses in Vibrionaceae: a combined approach using molecules and physiology. Int J Syst Bacteriol 53:2019–2026
Jones BW, Nishiguchi MK (2004) Counterillumination in the bobtail squid, Euprymna scolopes (Mollusca: Cephalopoda). Mar Biol 144:1151–1155
Ruby EG, Lee K-H (1998) The Vibrio fischeri–Euprymna scolopes light organ association: current ecological paradigms. Appl Environ Microbiol 64:805–812
Ruby EG (1996) Lessons from a cooperative, bacterial–animal association: the Vibrio fischeri–Euprymna scolopes light organ symbiosis. Annu Rev Microbiol 50:591–624
Nishiguchi MK (2002) Host recognition is responsible for symbiont composition in environmentally transmitted symbiosis. Microbiol Ecol 44:10–18
Kimbell JR, McFall-Ngai MJ, Roderick GK (2002) Two genetically distinct populations of bobtail squid, Euprymna scolopes, exist on the island of O’ahu. Pac Sci 56:347–355
Jones BW, Lopez JL, Huttenberg J, Nishiguchi MK (2006) Population structure between environmentally transmitted Vibrios and bobtail squids using nested clade analysis. Mol Ecol 15:4317–4329
Boettcher KJ, Ruby EG (1994) Occurrence of plasmid DNA in the sepiolid squid symbiont Vibrio fischeri. Current Microbiology 29:279–286
Lee K-H, Ruby EG (1994) Competition between Vibrio fischeri strains during initiation and maintenance of a light organ symbiosis. J Bacteriol 176:1985–1991
Nesis KN (1982) Cephalopods of the world. T.F.H., Neptune City, N.J
Nishiguchi MK, Ruby EG, McFall-Ngai MJ (1998) Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid–Vibrio symbioses. Appl Environ Microbiol 64:3209–3213
Nishiguchi MK (2000) Temperature affects species distribution in symbiotic populations of Vibrio. Appl Environ Microbiol 66:3550–3555
Jones BW, Maruyama A, Ouverney CC, Nishiguchi MK (2007) Spatial and temporal distribution of the Vibrionaceae in coastal waters of Hawaii, Australia, and France. Microbiol Ecol 54:314–323
Fidopiastis PM, Boletzky Sv, Ruby EG (1998) A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J Bacteriol 180:59–64
Kaspar CW, Tamplin ML (1993) Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl Environ Microbiol 59:2425–2429
Marco-Noales E, Biosca EG, Amaro C (1999) Effects of salinity and temperature on long-term survival of the eel pathogen Vibrio vulnificus biotype 2 (serovar E). Appl Environ Microbiol 65:1117–1126
Amaro C, Biosca EG, Fouz B, Alcaide E, Esteve C (1995) Evidence that water transmits Vibrio vulnificus biotype 2 infections to eels. Appl Environ Microbiol 61:1133–1137
Motes ML, DePaola A, Cook DW, Veazey JE, Hunsucker JC, Garthright WE, Blodgett RJ, Chirtel SJ (1998) Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl Environ Microbiol 64:1459–1465
Jiang SC (2001) Vibrio cholerae in recreational beach waters and tributaries of Southern California. Hydrobiol 460:157–164
Jiang SC, Fu W (2001) Seasonal abundance and distribution of Vibrio cholerae in coastal waters quantified by a 16S–23S intergenic spacer probe. Microbiol Ecol 42:540–548
Louis VR, Russek-Cohen E, Choopun N, Rivera ING, Gangle B, Jiang SC, Rubin A, Patz JA, Huq A, Colwell RR (2003) Predictability of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol 69:2773–2785
Hijarrubia MJ, Lazaro B, Sunen E, Fernandez-Astorga A (1996) Survival of Vibrio vulnificus under pH, salinity and temperature combined stress. Food Microbiol 13:193–199
Hoff KA (1989) Survival of Vibrio anguillarum and Vibrio salmonicida at different salinities. Appl Environ Microbiol 55:1775–1786
Wolf PW, Oliver JD (1992) Temperature effects on the viable but non-culturable state of Vibrio vulnificus. FEMS Microbiol Ecol 10:33–39
Bordas MA, Balebona MC, Zorrilla I, Borrego JJ, Morinigo MA (1996) Kinetics of adhesion of selected fish-pathogenic Vibrio strains to skin mucus of Gilt-Head Sea Bream (Sparus aurata L.). Appl Environ Microbiol 62:3650–3654
Xu C, Ren H, Wang S, Peng X (2004) Proteomic analysis of salt-sensitive outer membrane proteins of Vibrio parahaemolyticus. Res Microbiol 155:835–842
Xu C, Wang S, Ren H, Lin X, Wu L, Peng X (2005) Proteomic analysis on the expression of outer membrane proteins of Vibrio alginolyticus at different sodium concentrations. Proteomics 5:3142–3152
Sleator RD, Hill C (2001) Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol Rev 26:49–71
Nealson KH (1978) Isolation, identification, and manipulation of luminous bacteria. Methods Enzymol 57:153–165
Dunlap PV (1989) Regulation of luminescence by cyclic AMP in cya-like and crp-like mutants Vibrio fischeri. J Bacteriol 171:1199–1202
Lupp C, Hancock REW, Ruby EG (2002) The Vibrio fischeri sapABCDF locus is required for normal growth, both in culture and in symbiosis. Arch Microbiol 179:57–65
Nishiguchi MK, Ruby EG, McFall-Ngai MJ (1997) Phenotypic bioluminescence as an indicator of competitive dominance in the Euprymna–Vibrio symbiosis. In: Hastings JW, Kricka JW, Stanley PE (eds) Bioluminescence and chemiluminescence: molecular reporting with photons. Wiley, New York, NY, pp 123–126
Nishiguchi MK, Jones BW (2004) Microbial biodiversity within the Vibrionaceae. In: Seckback J (ed) Origins, evolution, and the biodiversity of microbial life. Kluwer, Dordrecht, The Netherlands, pp 531–548
Nishiguchi MK, Lopez JE, von Boletzky S (2004) Enlightenment of old ideas from new investigations: the evolution of bacteriogenic light organs in squids. Evol Dev 6:41–49
Atlas RM, Bartha R (1998) Microbial ecology: fundamentals and applications. Cummings, Menlo Park, CA
Yetinson T, Shilo M (1979) Seasonal and geographic distribution of luminous bacteria in the eastern Mediterranean Sea and the Gulf of Elat. Appl Environ Microbiol 37:1230–1238
O’Brien CH, Sizemore RK (1979) Distribution of the luminous bacterium Beneckea harveyi in a semitropical estuarine environment. Appl Environ Microbiol 38:928–933
Ruby EG, Nealson KH (1978) Seasonal changes in the species composition of luminous bacteria in nearshore seawater. Limnol Oceangr 23:530–533
Shilo M, Yetinson T (1979) Physiological characteristics underlying the distribution patterns of luminous bacteria in the Mediterranean Sea and the Gulf of Elat. Appl Environ Microbiol 38:577–584
Lee K-H, Ruby EG (1994) Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl Environ Microbiol 60:1565–1571
White D (2000) The physiology and biochemistry of the Prokaryotes. Oxford University Press, Oxford, UK
Norman MD, Lu CC (1997) Redescription of the southern dumpling squid Euprymna tasmanica and a revision of the genus Euprymna (Cephalopoda: Sepiolidae). J Mar Biol Assoc UK 77:1109–1137
Nateewathana A (1997) The Sepiolidae (Cephalopoda) of the Andaman Sea, Thailand, with description of Euprymna hyllebergi sp. nov. Phuket Mar Biol Cent Spec Pub 17:465–481
Rispe C, Moran NA (2000) Accumulation of deleterious mutations in endosymbionts: Muller’s ratchet with two levels of selection. Am Nat 156:425–441
Moran NA (1996) Accelerated evolution and Muller’s ratchet in endosymbiotic bacterial. Proc Nat Acad Sci USA 93:2873–2878
McFall-Ngai MJ (1999) Consequences of evolving with bacterial symbionts: insights from the squid–Vibrio association. Annu Rev Ecol Syst 30:235–256
Bennett AF, Lenski RE (1999) Experimental evolution and its role in evolutionary physiology. Am Zool 39:346–362
Giovannoni SJ, Sting U (2005) Molecular diversity and ecology of microbial plankton. Nature Rev Genet 437:343–348
Odic D, Turk V, Stopar S (2007) Environmental stress determines the quality of bacterial lysate and its utilization efficiency in a simple microbial loop. Microbiol Ecol 53:639–649
Vattanaviboon P, Mongkolsuk S (2001) Unusual adaptive, cross-protection response and growth phase resistance against peroxide killing in a bacterial shrimp pathogen, Vibrio harveyi. FEMS Microbiol Lett 47:111–116
Okutani T, Horita E (1987) Identity of Euprymna berryi Sasaki, 1929 (Cephalopoda: Sepiolidae). Venus 46:91–107
Acknowledgments
This work was supported in part by grants from the National Science Foundation Population Biology program (DEB-0316516) and the National Institutes of Health (SO6 GM008136-32S2-1) to M.K.N. W. Soto and J. Gutierrez were both supported by the NIH-MBRS RISE program (GM-61222-01) and the Howard Hughes Medical Institute (52005881). The authors would also like to thank A. Burris and J.G. Easterling (MBRS-RISE) for additional help with this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soto, W., Gutierrez, J., Remmenga, M.D. et al. Salinity and Temperature Effects on Physiological Responses of Vibrio fischeri from Diverse Ecological Niches. Microb Ecol 57, 140–150 (2009). https://doi.org/10.1007/s00248-008-9412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-008-9412-9