Abstract
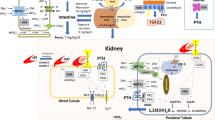
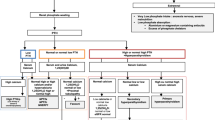
Phosphorus, predominantly in the form of inorganic phosphate PO4−3, has many essential physiological functions. In the skeleton, phosphate and calcium form the mineral component and phosphate is also essential in regulating function of skeletal cells. Considerable advances have been made in our understanding of phosphate homeostasis since the recognition of fibroblast growth factor-23 (FGF23) as a bone-derived phosphaturic hormone. This second part of a two-part review of disorders of phosphate homeostasis in children covers hypophosphatemic and hyperphosphatemic disorders that are of interest to the pediatric radiologist, emphasizing, but not limited to, those related to abnormalities of FGF23 signaling.









Similar content being viewed by others
References
ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348
Levine BS, Kleeman CR, Felsenfeld AJ (2009) The journey from vitamin D-resistant rickets to the regulation of renal phosphate transport. Clin J Am Soc Nephrol 4:1866–1877
Christov M, Jüppner H (2018) Phosphate homeostasis disorders. Best Pract Res Clin Endocrinol Metab 32:685–706
Koumakis E, Cormier C, Roux C et al (2020) The causes of hypo- and hyperphosphatemia in humans. Calcif Tissue Int 108:41–73
Michigami T, Ozono K (2019) Roles of phosphate in skeleton. Front Endocrinol 10:180
Razali NN, Hwu TT, Thilakavathy K (2015) Phosphate homeostasis and genetic mutations of familial hypophosphatemic rickets. J Pediatr Endocrinol Metab 28:1009–1017
Bitzan M, Goodyer PR (2019) Hypophosphatemic rickets. Pediatr Clin N Am 66:179–207
Lambert AS, Linglart A (2018) Hypocalcaemic and hypophosphatemic rickets. Best Pract Res Clin Endocrinol Metab 32:455–476
Whyte MP, Schranck FW, Armamento-Villareal R (1996) X-linked hypophosphatemia: a search for gender, race, anticipation, or parent of origin effects on disease expression in children. J Clin Endocrinol Metab 81:4075–4080
Liu S, Guo R, Simpson LG et al (2003) Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278:37419–37426
Imel EA (2020) Congenital conditions of hypophosphatemia in children. Calcif Tissue Int 108:74–90
Carpenter TO, Imel EA, Holm IA et al (2011) A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res 26:1381–1388
Gohil A, Imel EA (2019) FGF23 and associated disorders of phosphate wasting. Pediatr Endocrinol Rev 17:17–34
Bacchetta J, Bardet C, Prié D (2020) Physiology of FGF23 and overview of genetic diseases associated with renal phosphate wasting. Metabolism 103s:153865
Beck-Nielsen SS, Brixen K, Gram J et al (2013) High bone mineral apparent density in children with X-linked hypophosphatemia. Osteoporos Int 24:2215–2221
Oliveri MB, Cassinelli H, Bergadá C et al (1991) Bone mineral density of the spine and radius shaft in children with X-linked hypophosphatemic rickets (XLH). Bone Miner 12:91–100
Reid IR, Murphy WA, Hardy DC et al (1991) X-linked hypophosphatemia: skeletal mass in adults assessed by histomorphometry, computed tomography, and absorptiometry. Am J Med 90:63–69
Shore RM, Langman CB, Poznanski AK (2000) Lumbar and radial bone mineral density in children and adolescents with X-linked hypophosphatemia: evaluation with dual X-ray absorptiometry. Skelet Radiol 29:90–93
Rauch F (2006) Material matters: a mechanostat-based perspective on bone development in osteogenesis imperfecta and hypophosphatemic rickets. J Musculoskelet Neuronal Interact 6:142–146
Frost HM (1987) The mechanostat: a proposed pathogentic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner 2:73–85
Arango Sancho P (2020) Complications of phosphate and vitamin D treatment in X-linked hypophosphataemia. Adv Ther 37:105–112
Colares Neto GP, Ide Yamauchi F, Hueb Baroni R et al (2019) Nephrocalcinosis and nephrolithiasis in X-linked hypophosphatemic rickets: diagnostic imaging and risk factors. J Endocr Soc 3:1053–1061
Carpenter TO, Imel EA, Ruppe MD et al (2014) Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest 124:1587–1597
Carpenter TO, Whyte MP, Imel EA et al (2018) Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med 378:1987–1998
Imel EA, Glorieux FH, Whyte MP et al (2019) Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet 393:2416–2427
Insogna KL, Briot K, Imel EA et al (2018) A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res 33:1383–1393
Santos Rodriguez F (2020) X-linked hypophosphataemic rickets and growth. Adv Ther 37:55–61
Whyte MP, Carpenter TO, Gottesman GS et al (2019) Efficacy and safety of burosumab in children aged 1-4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol 7:189–199
Athonvarangkul D, Insogna KL (2021) New therapies for hypophosphatemia-related to FGF23 excess. Calcif Tissue Int 108:143–157
Fukumoto S (2021) FGF23-related hypophosphatemic rickets/osteomalacia: diagnosis and new treatment. J Mol Endocrinol 66:R57–R65
Harada D, Ueyama K, Oriyama K et al (2021) Switching from conventional therapy to burosumab injection has the potential to prevent nephrocalcinosis in patients with X-linked hypophosphatemic rickets. J Pediatr Endocrinol Metab 34:791–798
Imel EA (2021) Burosumab for pediatric X-linked hypophosphatemia. Curr Osteoporos Rep 19:271–277
Bacchetta J, Rothenbuhler A, Gueorguieva I et al (2021) X-linked hypophosphatemia and burosumab: practical clinical points from the French experience. Joint Bone Spine 88:105208
Ferreira CR, Kintzinger K, Hackbarth ME et al (2021) Ectopic calcification and hypophosphatemic rickets: natural history of ENPP1 and ABCC6 deficiencies. J Bone Miner Res 36:2193–2202
Ferreira CR, Hackbarth ME, Ziegler SG et al (2021) Prospective phenotyping of long-term survivors of generalized arterial calcification of infancy (GACI). Genet Med 23:396–407
Höppner J, Kornak U, Sinningen K et al (2021) Autosomal recessive hypophosphatemic rickets type 2 (ARHR2) due to ENPP1-deficiency. Bone 153:116111
Stern R, Levi DS, Gales B et al (2021) Correspondence on "prospective phenotyping of long-term survivors of generalized arterial calcification of infancy (GACI)" by Ferreira et al. Genet Med 23:2006–2007
Chong WH, Molinolo AA, Chen CC, Collins MT (2011) Tumor-induced osteomalacia. Endocr Relat Cancer 18:R53–R77
Drezner MK (2001) Tumor-induced osteomalacia. Rev Endocr Metab Disord 2:175–186
Farrow EG, White KE (2009) Tumor-induced osteomalacia. Expert Rev Endocrinol Metab 4:435–442
Florenzano P, Hartley IR, Jimenez M et al (2020) Tumor-induced osteomalacia. Calcif Tissue Int 108:128–142
Yin Z, Du J, Yu F, Xia W (2018) Tumor-induced osteomalacia. Osteoporos Sarcopenia 4:119–127
Harrison HE (1973) Oncogenous rickets: possible elaboration by a tumor of a humoral substance inhibiting tubular reabsorption of phosphate. Pediatrics 52:432–434
Jan De Beur SM, Finnegan RB, Vassiliadis J et al (2002) Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res 17:1102–1110
Shimada T, Mizutani S, Muto T et al (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98:6500–6505
Folpe AL, Fanburg-Smith JC, Billings SD et al (2004) Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol 28:1–30
Jiang Y, Xia WB, Xing XP et al (2012) Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: report of 39 cases and review of the literature. J Bone Miner Res 27:1967–1975
Chande S, Bergwitz C (2018) Role of phosphate sensing in bone and mineral metabolism. Nat Rev Endocrinol 14:637–655
Lee JC, Su SY, Changou CA et al (2016) Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod Pathol 29:1335–1346
Minisola S, Peacock M, Fukumoto S et al (2017) Tumour-induced osteomalacia. Nat Rev Dis Primers 3:17044
Jung GH, Kim JD, Cho Y et al (2010) A 9-month-old phosphaturic mesenchymal tumor mimicking the intractable rickets. J Pediatr Orthop B 19:127–132
Fernández-Cooke E, Cruz-Rojo J, Gallego C et al (2015) Tumor-induced rickets in a child with a central giant cell granuloma: a case report. Pediatrics 135:e1518–e1523
Crossen SS, Zambrano E, Newman B et al (2017) Tumor-induced osteomalacia in a 3-year-old with unresectable central giant cell lesions. J Pediatr Hematol Oncol 39:e21–e24
Ma GM, Chow JS, Taylor GA (2019) Review of paraneoplastic syndromes in children. Pediatr Radiol 49:534–550
Duet M, Kerkeni S, Sfar R et al (2008) Clinical impact of somatostatin receptor scintigraphy in the management of tumor-induced osteomalacia. Clin Nucl Med 33:752–756
Jan de Beur SM, Streeten EA, Civelek AC et al (2002) Localisation of mesenchymal tumours by somatostatin receptor imaging. Lancet 359:761–763
Nguyen BD, Wang EA (1999) Indium-111 pentetreotide scintigraphy of mesenchymal tumor with oncogenic osteomalacia. Clin Nucl Med 24:130–131
El-Maouche D, Sadowski SM, Papadakis GZ et al (2016) 68Ga-DOTATATE for tumor localization in tumor-induced osteomalacia. J Clin Endocrinol Metab 101:3575–3581
Florenzano P, Gafni RI, Collins MT (2017) Tumor-induced osteomalacia. Bone Rep 7:90–97
Rayamajhi SJ, Yeh R, Wong T et al (2019) Tumor-induced osteomalacia — current imaging modalities and a systematic approach for tumor localization. Clin Imaging 56:114–123
Andreopoulou P, Dumitrescu CE, Kelly MH et al (2011) Selective venous catheterization for the localization of phosphaturic mesenchymal tumors. J Bone Miner Res 26:1295–1302
Hesse E, Rosenthal H, Bastian L (2007) Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N Engl J Med 357:422–424
de Castro LF, Ovejero D, Boyce AM (2020) Diagnosis of endocrine disease: mosaic disorders of FGF23 excess: fibrous dysplasia/McCune-Albright syndrome and cutaneous skeletal hypophosphatemia syndrome. Eur J Endocrinol 182:R83–R99
Riminucci M, Collins MT, Fedarko NS et al (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112:683–692
Collins MT, Singer FR, Eugster E (2012) McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis 7:S4
Kuznetsov SA, Cherman N, Riminucci M et al (2008) Age-dependent demise of GNAS-mutated skeletal stem cells and "normalization" of fibrous dysplasia of bone. J Bone Miner Res 23:1731–1740
Bhattacharyya N, Wiench M, Dumitrescu C et al (2012) Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res 27:1132–1141
Aschinberg LC, Solomon LM, Zeis PM et al (1977) Vitamin D-resistant rickets associated with epidermal nevus syndrome: demonstration of a phosphaturic substance in the dermal lesions. J Pediatr 91:56–60
Lim YH, Ovejero D, Sugarman JS et al (2014) Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet 23:397–407
Ovejero D, Lim YH, Boyce AM et al (2016) Cutaneous skeletal hypophosphatemia syndrome: clinical spectrum, natural history, and treatment. Osteoporos Int 27:3615–3626
Heike CL, Cunningham ML, Steiner RD et al (2005) Skeletal changes in epidermal nevus syndrome: does focal bone disease harbor clues concerning pathogenesis? Am J Med Genet 139A:67–77
Pitt MJ (2002) Rickets and osteomalacia. In: Resnick D (ed) Diagnosis of bone and joint disorders, 4th edn. Saunders, Philadelphia, pp 1901–1946
White KE, Cabral JM, Davis SI et al (2005) Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet 76:361–367
Wagner CA, Rubio-Aliaga I, Hernando N (2017) Renal phosphate handling and inherited disorders of phosphate reabsorption: an update. Pediatr Nephrol 34:549–559
Tiosano D, Hochberg Z (2009) Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab 27:392–401
Ito N, Fukumoto S (2021) Congenital hyperphosphatemic conditions caused by the deficient activity of FGF23. Calcif Tissue Int 108:104–115
Roberts MS, Burbelo PD, Egli-Spichtig D et al (2018) Autoimmune hyperphosphatemic tumoral calcinosis in a patient with FGF23 autoantibodies. J Clin Invest 128:5368–5373
Resnick D (2002) Soft tissue disorders. In: Resnick D (ed) Diagnosis of bone and joint disorders, 4th edn. W. B. Saunders, Philadelphia, pp 4635–4695
Martinez S, Vogler JB 3rd, Harrelson JM, Lyles KW (1990) Imaging of tumoral calcinosis: new observations. Radiology 174:215–222
Olsen KM, Chew FS (2006) Tumoral calcinosis: pearls, polemics, and alternative possibilities. Radiographics 26:871–885
Boyce AM, Lee AE, Roszko KL, Gafni RI (2020) Hyperphosphatemic tumoral calcinosis: pathogenesis, clinical presentation, and challenges in management. Front Endocrinol 11:293
Ramnitz MS, Gourh P, Goldbach-Mansky R et al (2016) Phenotypic and genotypic characterization and treatment of a cohort with familial tumoral calcinosis/hyperostosis-hyperphosphatemia syndrome. J Bone Miner Res 31:1845–1854
Folsom LJ, Imel EA (2015) Hyperphosphatemic familial tumoral calcinosis: genetic models of deficient FGF23 action. Curr Osteoporos Rep 13:78–87
Clarke E, Swischuk LE, Hayden CK Jr (1984) Tumoral calcinosis, diaphysitis, and hyperphosphatemia. Radiology 151:643–646
Talab YA, Mallouh A (1988) Hyperostosis with hyperphosphatemia: a case report and review of the literature. J Pediatr Orthop 8:338–341
Narchi H (1997) Hyperostosis with hyperphosphatemia: evidence of familial occurrence and association with tumoral calcinosis. Pediatrics 99:745–748
Frishberg Y, Ito N, Rinat C et al (2007) Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res 22:235–242
Slavin RE, Wen J, Barmada A (2012) Tumoral calcinosis — a pathogenetic overview: a histological and ultrastructural study with a report of two new cases, one in infancy. Int J Surg Pathol 20:462–473
Finer G, Price HE, Shore RM et al (2014) Hyperphosphatemic familial tumoral calcinosis: response to acetazolamide and postulated mechanisms. Am J Med Genet A 164A:1545–1549
Ito E, Konno Y, Toki T, Terui K (2010) Molecular pathogenesis in diamond-Blackfan anemia. Int J Hematol 92:413–418
Vervloet MG (2020) FGF23 measurement in chronic kidney disease: what is it really reflecting? Clin Chim Acta 505:160–166
Acknowledgments
I would like to thank Aaron L. Friedman, MD, for his helpful advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shore, R.M. Disorders of phosphate homeostasis in children, part 2: hypophosphatemic and hyperphosphatemic disorders. Pediatr Radiol 52, 2290–2305 (2022). https://doi.org/10.1007/s00247-022-05373-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05373-z