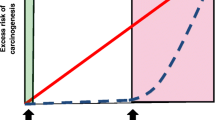
Abstract
Neonates represent a unique subset of the pediatric population that requires special attention and careful thought when implementing advanced cross-sectional imaging with CT or MRI. The ionizing radiation associated with CT and the sedation/anesthesia occasionally required for MRI present risks that must be balanced against the perceived benefit of the imaging examination in the unique and particularly susceptible neonatal population. We review the perceived risks of ionizing radiation and the more concrete risks of sedation/anesthesia in term and preterm neonates in the context of an imaging paradigm. When the expected diagnostic yield from CT and MRI is similar, and sedation is required for MRI but not for CT, CT likely has the higher benefit-to-risk ratio in the neonate. However, despite the risks, the most appropriate imaging modality should always be chosen after thoughtful consideration is given to each unique patient and informed discussions including radiology, anesthesia, neonatology and the parents/caregivers are pursued.
Similar content being viewed by others
References
Callahan MJ, MacDougall RD, Bixby SD et al (2018) Ionizing radiation from computed tomography versus anesthesia for magnetic resonance imaging in infants and children: patient safety considerations. Pediatr Radiol 48:21–30
Bartley K, Metayer C, Selvin S et al (2010) Diagnostic X-rays and risk of childhood leukaemia. Int J Epidemiol 39:1628–1637
Brenner D, Elliston C, Hall E, Berdon W (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 176:289–296
Brenner DJ (2002) Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol 32:228–231
Brenner DJ, Doll R, Goodhead DT et al (2003) Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A 100:13761–13766
Chodick G, Ronckers CM, Shalev V, Ron E (2007) Excess lifetime cancer mortality risk attributable to radiation exposure from computed tomography examinations in children. Isr Med Assoc J 9:584–587
Mathews JD, Forsythe AV, Brady Z et al (2013) Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346:f2360
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Preston DL, Cullings H, Suyama A et al (2008) Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst 100:428–436
Preston DL, Ron E, Tokuoka S et al (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168:1–64
International Commission on Radiological Protection (2007) The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 37:2–4
Frush DP (2011) CT dose and risk estimates in children. Pediatr Radiol 41:483–487
Strauss KJ, Goske MJ (2011) Estimated pediatric radiation dose during CT. Pediatr Radiol 41:472–482
Strauss KJ, Goske MJ, Frush DP et al (2009) Image Gently vendor summit: working together for better estimates of pediatric radiation dose from CT. AJR Am J Roentgenol 192:1169–1175
Strauss KJ, McKenney SE, Brady SL (2020) Improved estimates of trunk and head CT radiation dose: development of size-specific dose estimate. J Am Coll Radiol 17:560–562
Little MP, Wakeford R, Tawn EJ et al (2009) Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology 251:6–12
American Association of Physicists in Medicine (2011) Position statement on radiation risks from medical imaging procedures. https://www.aapm.org/org/policies/details.asp?id=318&type=PP¤t=true. Accessed 19 Feb 2021
Hendee WR, O'Connor MK (2012) Radiation risks of medical imaging: separating fact from fantasy. Radiology 264:312–321
Hricak H, Brenner DJ, Adelstein SJ et al (2011) Managing radiation use in medical imaging: a multifaceted challenge. Radiology 258:889–905
World Health Organization (2016) Communicating radiation risks in paediatric imaging: information to support health care discussions about benefit and risk. World Health Organization, Geneva, pp 1–86
Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII phase. National Academies Press, Washington, DC
Johnson JN, Hornik CP, Li JS et al (2014) Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation 130:161–167
American Assocation of Physicists in Medicine (2021) The alliance for quality computed tomography. https://www.aapm.org/pubs/CTProtocols/. Accessed 19 Feb 2021
Gottumukkala RV, Kalra MK, Tabari A et al (2019) Advanced CT techniques for decreasing radiation dose, reducing sedation requirements, and optimizing image quality in children. Radiographics 39:709–726
Huda W, Lieberman KA, Chang J, Roskopf ML (2004) Patient size and X-ray technique factors in head computed tomography examinations. I. Radiation doses. Med Phys 31:588–594
MacDougall RD, Kleinman PL, Yu L, Lee EY (2016) Pediatric thoracic CT angiography at 70 kV: a phantom study to investigate the effects on image quality and radiation dose. Pediatr Radiol 46:1114–1119
Siegel MJ, Hildebolt C, Bradley D (2013) Effects of automated kilovoltage selection technology on contrast-enhanced pediatric CT and CT angiography. Radiology 268:538–547
Kalender WA, Deak P, Kellermeier M et al (2009) Application- and patient size-dependent optimization of X-ray spectra for CT. Med Phys 36:993–1007
Kino A, Zucker EJ, Honkanen A et al (2019) Ultrafast pediatric chest computed tomography: comparison of free-breathing vs. breath-hold imaging with and without anesthesia in young children. Pediatr Radiol 49:301–307
Bodelle B, Fischbach C, Booz C et al (2017) Free-breathing high-pitch 80 kVp dual-source computed tomography of the pediatric chest: image quality, presence of motion artifacts and radiation dose. Eur J Radiol 89:208–214
Paterson N, Waterhouse P (2011) Risk in pediatric anesthesia. Paediatr Anaesth 21:848–857
Cravero JP (2009) Risk and safety of pediatric sedation/anesthesia for procedures outside the operating room. Curr Opin Anaesthesiol 22:509–513
Cravero JP, Beach ML, Blike GT et al (2009) The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the pediatric sedation research consortium. Anesth Analg 108:795–804
Greenberg SB (2011) Rebalancing the risks of computed tomography and magnetic resonance imaging. Pediatr Radiol 41:951–952
Masaracchia MM, Tsapakos MJ, McNulty NJ, Beach ML (2017) Changing the paradigm for diagnostic MRI in pediatrics: don't hold your breath. Paediatr Anaesth 27:880–884
Cote CJ, Zaslavsky A, Downes JJ et al (1995) Postoperative apnea in former preterm infants after inguinal herniorrhaphy. A combined analysis. Anesthesiology 82:809–822
Parad RB (2018) Non-sedation of the neonate for radiologic procedures. Pediatr Radiol 48:524–530
Havidich JE, Beach M, Dierdorf SF et al (2016) Preterm versus term children: analysis of sedation/anesthesia adverse events and longitudinal risk. Pediatrics 137:e20150463
Brown ML, DiNardo JA, Nasr VG (2020) Anesthesia in pediatric patients with congenital heart disease undergoing noncardiac surgery: defining the risk. J Cardiothorac Vasc Anesth 34:470–478
Murat I, Constant I, Maud'huy H (2004) Perioperative anaesthetic morbidity in children: a database of 24,165 anaesthetics over a 30-month period. Paediatr Anaesth 14:158–166
Tay CL, Tan GM, Ng SB (2001) Critical incidents in paediatric anaesthesia: an audit of 10,000 anaesthetics in Singapore. Paediatr Anaesth 11:711–718
Hoffman GM (2008) Outcomes of pediatric anesthesia. Semin Pediatr Surg 17:141–151
Williams GD, Maan H, Ramamoorthy C et al (2010) Perioperative complications in children with pulmonary hypertension undergoing general anesthesia with ketamine. Paediatr Anaesth 20:28–37
Jevtovic-Todorovic V, Hartman RE, Izumi Y et al (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882
Rappaport B, Mellon RD, Simone A, Woodcock J (2011) Defining safe use of anesthesia in children. N Engl J Med 364:1387–1390
Young C, Jevtovic-Todorovic V, Qin YQ et al (2005) Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol 146:189–197
Flick RP, Katusic SK, Colligan RC et al (2011) Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128:e1053–e1061
Wilder RT, Flick RP, Sprung J et al (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804
McCann ME, de Graaff JC, Dorris L et al (2019) Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393:664–677
Koo E, Oshodi T, Meschter C et al (2014) Neurotoxic effects of dexmedetomidine in fetal cynomolgus monkey brains. J Toxicol Sci 39:251–262
Liao Z, Cao D, Han X et al (2014) Both JNK and P38 MAPK pathways participate in the protection by dexmedetomidine against isoflurane-induced neuroapoptosis in the hippocampus of neonatal rats. Brain Res Bull 107:69–78
Nasr VG, Davis JM (2015) Anesthetic use in newborn infants: the urgent need for rigorous evaluation. Pediatr Res 78:2–6
Antonov NK, Ruzal-Shapiro CB, Morel KD et al (2017) Feed and wrap MRI technique in infants. Clin Pediatr 56:1095–1103
Heller BJ, Yudkowitz FS, Lipson S (2017) Can we reduce anesthesia exposure? Neonatal brain MRI: swaddling vs. sedation, a national survey. J Clin Anesth 38:119–122
Templeton LB, Norton MJ, Goenaga-Diaz EJ et al (2020) Experience with a "feed and swaddle" program in infants up to six months of age. Acta Anaesthesiol Scand 64:63–68
Jaimes C, Kirsch JE, Gee MS (2018) Fast, free-breathing and motion-minimized techniques for pediatric body magnetic resonance imaging. Pediatr Radiol 48:1197–1208
Janos S, Schooler GR, Ngo JS, Davis JT (2019) Free-breathing unsedated MRI in children: justification and techniques. J Magn Reson Imaging 50:365–376
Kozak BM, Jaimes C, Kirsch J, Gee MS (2020) MRI techniques to decrease imaging times in children. Radiographics 40:485–502
Ahmad R, Hu HH, Krishnamurthy R, Krishnamurthy R (2018) Reducing sedation for pediatric body MRI using accelerated and abbreviated imaging protocols. Pediatr Radiol 48:37–49
Lee JH, Choi YH, Cheon JE et al (2015) Improved abdominal MRI in non-breath-holding children using a radial k-space sampling technique. Pediatr Radiol 45:840–846
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schooler, G.R., Cravero, J.P. & Callahan, M.J. Assessing and conveying risks and benefits of imaging in neonates using ionizing radiation and sedation/anesthesia. Pediatr Radiol 52, 616–621 (2022). https://doi.org/10.1007/s00247-021-05138-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05138-0