Abstract
Background
Pancreatic neuroendocrine tumors are not included in the diagnostic criteria for tuberous sclerosis complex, although an association has been described.
Objective
To investigate the association of pancreatic neuroendocrine tumor in children and young adults with tuberous sclerosis complex and define MRI characteristics of the tumor.
Materials and methods
We retrospectively evaluated the abdominal MRI scans of 55 children and young adults with tuberous sclerosis complex for the presence of a pancreatic mass. The scans were performed over a period of 7 years to monitor renal pathology. We obtained each patient’s clinical history and treatment protocol from the hospital’s electronic medical records.
Results
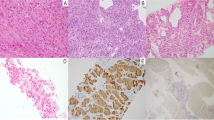
A solid pancreatic mass was identified in 5/55 (9%, 95% confidence interval [CI] 3–20%) patients (4 male) with a mean age of 12.6 years. Four of the lesions were located in the pancreatic tail and one in the pancreatic body. All of the lesions were solid, ovoid and well demarcated, with a mean diameter of 3.1 cm. The masses uniformly demonstrated T1 and T2 prolongation, but their diffusion behavior and post-contrast enhancement varied. The two surgically resected lesions were synaptophysin (+) non-functional pancreatic neuroendocrine tumors on pathology. Two of the patients who did not have surgery were treated with everolimus; one of the lesions has shown interval decrease in size and the other has remained stable.
Conclusion
Pancreatic tumor is relatively common in children and young adults with tuberous sclerosis complex.





Similar content being viewed by others
References
Sancak O, Nellist M, Goedbloed M et al (2005) Mutational analysis of the TSC1 and TSC2 genes in diagnostic setting: genotype-phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complex. Eur J Hum Genet 13:731–741
Manning BD, Tee AR, Logsdon MN et al (2002) Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10:151–162
Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group (2013) Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49:243–254
Larson AM, Hedgire SS, Deshpande V et al (2012) Pancreatic neuroendocrine tumors in patients with tuberous sclerosis complex. Clin Genet 82:558–563
Diaz Diaz D, Ibarrola C, Gomez Sanz R et al (2012) Neuroendocrine tumor of the pancreas in a patient with tuberous sclerosis: a case report and review of the literature. Int J Surg Pathol 20:390–395
Francalanci P, Diomedi-Camassei F, Purificato C et al (2003) Malignant pancreatic endocrine tumor in a child with tuberous sclerosis. Am J Surg Pathol 27:1386–1389
Ilgren EB, Westmoreland D (1984) Tuberous sclerosis: unusual associations in four cases. J Clin Pathol 37:272–278
Merritt JL 2nd, Davis DM, Pittelkow MR et al (2006) Extensive acrochordons and pancreatic islet-cell tumors in tuberous sclerosis associated with TSC2 mutations. Am J Med Genet 140:1669–1672
Sreenarasimhaiah J, Armstrong LA, Tang SJ et al (2009) Pancreatic somatostatinoma and tuberous sclerosis: case report and exceedingly rare association. Gastrointest Endosc 69:379–381
Verhoef S, van Diemen-Steenvoorde R, Akkersdijk WL et al (1999) Malignant pancreatic tumor within the spectrum of tuberous sclerosis complex in childhood. Eur J Pediatr 158:284–287
Bombardieri R, Moavero R, Roberto D et al (2013) Pancreatic neuroendocrine tumor in a child with a tuberous sclerosis complex 2 (TSC2) mutation. Endocr Pract 19:e124–e128
Dworakowska D, Grossman AB (2009) Are neuroendocrine tumours a feature of tuberous sclerosis? A systematic review. Endocr Relat Cancer 16:45–58
Eledrisi MS, Stuart CA, Alshanti M (2002) Insulinoma in a patient with tuberous sclerosis: is there an association? Endocr Pract 8:109–112
Van den Akker M, Angelini P, Taylor G et al (2012) Malignant pancreatic tumors in children: a single institution series. J Pediatr Surg 47:681–687
Roman-Goldstein S, Mitchell P, Crossen JR et al (1995) The association between tuberous sclerosis and insulinoma. AJNR Am J Neuroradiol 16:1543–1544
Wiedenmann B, Pavel M, Kos-Kudla B (2011) From targets to treatments: a review of molecular targets in pancreatic neuroendocrine tumors. Neuroendocrinology 94:177–190
Oberstein PE, Remotti H, Saif MW et al (2012) Pancreatic neuroendocrine tumors: entering a new era. JOP 13:169–173
Hruban RH, Pitman MB, Klimstra DS (2007) Tumors of the pancreas. In: Silverberg SG, Sobin LH (eds) AFIP atlas of tumor pathology, series 4, vol 6. American Registry of Pathology, Washington, DC
Panzuto F, Rinzivillo M, Fazio N et al (2014) Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist 19:966–974
Yao JC, Fazio N, Singh S et al (2016) Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387:968–977
Buetow PC, Parrino TV, Buck JL et al (1995) Islet cell tumors of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am J Roentgenol 165:1175–1179
Lewis RB, Lattin GE, Paal E (2010) Pancreatic endocrine tumors: radiologic, clinicopathologic correlation. Radiographics 30:1445–1464
Reznek RH (2006) CT/MRI of neuroendocrine tumors. Cancer Imaging 31:163–177
Thoeni RF, Mueller-Lisse UG, Chan R et al (2000) Detection of small, functional islet cell tumors of the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology 214:483–490
Pereira JA, Rosado E, Bali M et al (2015) Pancreatic neuroendocrine tumors: correlation between histogram analysis of apparent diffusion coefficient maps and tumor grade. Abdom Imaging 40:3122–3128
Kim JY, Song JS, Park H et al (2014) Primary mesenchymal tumors of the pancreas: single-center experience over 16 years. Pancreas 43:959–968
Lassel EA, Rao R, Schwenke C et al (2014) Diffusion-weighted imaging of focal renal lesions: a meta-analysis. Eur Radiol 24:241–249
Kang SK, Huang WC, Pandharipande PV et al (2014) Solid renal masses: what the numbers tell us. AJR Am J Roentgenol 202:1196–1206
Tanaka H, Yoshida S, Fujii Y et al (2011) Diffusion-weighted magnetic resonance imaging in the differentiation of angiomyolipoma with minimal fat from clear cell renal cell carcinoma. Int J Urol 18:727–730
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Koc, G., Sugimoto, S., Kuperman, R. et al. Pancreatic tumors in children and young adults with tuberous sclerosis complex. Pediatr Radiol 47, 39–45 (2017). https://doi.org/10.1007/s00247-016-3701-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3701-0