Abstract
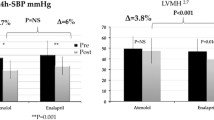
Hypertension after cardiothoracic surgery is common, often requiring pharmacologic management. The recommended first-line antihypertensives in pediatrics are angiotensin converting enzyme inhibitors. Captopril and enalapril are approved for infants and children; however, lisinopril is only approved for > 7 years of age. This study evaluated safety and efficacy of converting from captopril to lisinopril in patients utilizing a pre-defined conversion of 3 mg captopril to 1 mg lisinopril. This was a single center, retrospective study including patients less than 7 years of age admitted for cardiothoracic surgery who received both captopril and lisinopril from 01/01/2017 to 06/01/2022.The primary outcome was mean change in systolic blood pressure (SBP) from baseline for 72 h after conversion of captopril to lisinopril. A total of 99 patients were enrolled. There was a significant decrease in mean SBP (99.12 mmHg vs 94.86 mmHg; p = 0.007) with no difference in DBP (59.23 mmHg vs 61.95 mmHg; p = 0.07) after conversion to lisinopril. Of the 99 patients who were transitioned to lisinopril, 79 (80%) had controlled SBP, 20 (20%) remained hypertensive, 13 (13%) received an increase in their lisinopril dose, and 2 (2%) required an additional antihypertensive agent. There was a low overall rate of AKI (3%) and hyperkalemia (4%) respectively. This study demonstrates that utilizing lisinopril with a conversion rate of 3 mg of captopril to 1 mg of lisinopril was safe and effective for controlling hypertension in pediatric patients following cardiothoracic surgery.


Similar content being viewed by others
References
Greenberg JH, McArthur E, Thiessen-Philbrook H et al (2021) Long-term risk of hypertension after surgical repair of congenital heart disease in children. JAMA network open. 4(4):e215237
Stone ML, Kelly J, Mistry M et al (2018) Use of nicardipine after cardiac operations is safe in children regardless of age. Ann Thorac Surg 105(1):181–185
Magri P, Rao MA, Cangianiello S et al (1998) Early impairment of renal hemodynamic reserve in patients with asymptomatic heart failure is restored by angiotensin II antagonism. Circulation 98(25):2849–2854. https://doi.org/10.1161/01.CIR.98.25.2849
Ishikawa S, Miyauchi T, Sakai S et al (1995) Elevated levels of plasma endothelin-1 in young patients with pulmonary hypertension caused by congenital heart disease are decreased after successful surgical repair. J Thorac Cardiovasc Surg 110(1):271–273
Lang RE, Unger T, Ganten D et al (1985) Alpha atrial natriuretic peptide concentrations in plasma of children with congenital heart and pulmonary diseases. BMJ (Clin Res Ed) 291(6504):1241
Schrier RW, Abraham WT (1999) Hormones and hemodynamics in heart failure. N Engl J Med 341(8):577–585
Greenberg JH, Coca S, Parikh CR (2014) Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol 15:184
Cheung AT (2006) Exploring an optimum intra/postoperative management strategy for acute hypertension in the cardiac surgery patient. J Card Surg 21(Suppl 1):S8–S14
McEwen CC, Amir T, Qiu Y et al (2022) Morbidity and mortality in patients managed with high compared with low blood pressure targets during on-pump cardiac surgery: a systematic review and meta-analysis of randomized controlled trials. Can J Anaesth. 69(3):374–386
Vedel AG, Holmgaard F, Rasmussen LS et al (2018) High-target versus low-target blood pressure management during cardiopulmonary bypass to prevent cerebral injury in cardiac surgery patients: a randomized controlled trial. Circulation 137(17):1770–1780
Flynn Joseph T, Kaelber David C, Baker-Smith Carissa M et al (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140(3):e20171904. https://doi.org/10.1542/peds.2017-1904
Qbrelis (lisinopril) (1988) Azurity Pharmaceuticals Inc., Woburn, Massachusetts
Captopril (1980) Camber Pharmaceuticals Inc., Piscataway, New Jersey
Epaned (enalapril) (1985) Azurity Pharmaceuticals Inc., Woburn, Massachusetts
Gill TH, Hauter F, Pelter MA (1996) Conversions from captopril to lisinopril at a dosage ratio of 5:1 result in comparable control of hypertension. Ann Pharmacother 30(1):7–11
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120(4):c179–c184
Villa A, Vollemans M, De Moraes A, Sonis S (2021) Concordance of the WHO, RTOG, and CTCAE v4.0 grading scales for the evaluation of oral mucositis associated with chemoradiation therapy for the treatment of oral and oropharyngeal cancers. Support Care Cancer. 29:6061–8
Patil I (2021) Visualizations with statistical details: the “ggstatsplot” approach. J Open Source Softw 6(61):3167. https://doi.org/10.21105/joss.03167
Mirkin BL, Newman TJ (1985) Efficacy and safety of captopril in the treatment of severe childhood hypertension: report of the international collaborative study group. Pediatrics 75(6):1091–1100
Smeets NJ, Schreuder MF, Dalinghaus M et al (2020) Pharmacology of enalapril in children: a review. Drug Discovery Today 25(11):1957–1970
Wells T, Frame V, Soffer B et al (2002) Enalapril pediatric hypertension collaborative study group. A double-blind, placebo-controlled, dose-response study of the effectiveness and safety of enalapril for children with hypertension. J Clin Pharmacol 42(8):870–80
Hsu DT, Zak V, Mahony L et al (2010) Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation 122(4):333–340
Lewis AB, Chabot M (1993) The effect of treatment with angiotensin-converting enzyme inhibitors on survival of pediatric patients with dilated cardiomyopathy. Pediatr Cardiol 14:9–12
Schaefer F, Litwin M, Zachwieja J et al (2011) Efficacy and safety of valsartan compared to enalapril in hypertensive children: a 12-week, randomized, double-blind, parallel-group study. J Hypertens 29(12):2484–2490
Van Der Meulen M, Dalinghaus M, Burch M, Szatmari A et al (2018) Question 1: how safe are ACE inhibitors for heart failure in children? Arch Dis Child 103(1):106–109
Van der Meulen M, den Boer S, du Marchie Sarvaas GJ et al (2021) Predicting outcome in children with dilated cardiomyopathy: the use of repeated measurements of risk factors for outcome. ESC Heart Failure 8(2):1472–1481
El-Rachidi S, Larochelle JM, Morgan JA (2017) Pharmacists and pediatric medication adherence: bridging the gap. Hosp Pharm 52(2):124–131
Funding
The authors did not receive support or funding from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
JB and MA wrote the main manuscript text, and did the data collection. HZ and AB participated in project conceptualization and development, and performed final review of the manuscript. MG provided final review of the manuscript, performed statistical analysis of the collected data, and prepared table 1, and figures 1 and 2.
Corresponding author
Ethics declarations
Competing Interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to disclose for the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bransetter, J.W., Anderson, M., Zaki, H. et al. Captopril to Lisinopril Conversion in Pediatric Cardiothoracic Surgery Patients Less Than 7 Years of Age (RISE-7). Pediatr Cardiol 45, 394–400 (2024). https://doi.org/10.1007/s00246-023-03373-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03373-w