Abstract
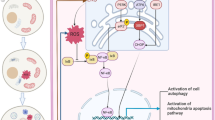
This study aimed to observe whether calcium oxalate (CaOx) crystals can induce the activation of endoplasmic reticulum (ER) stress in human renal cortex proximal tubule epithelial (HK-2) cells and to explore the regulatory of ER stress on the damage and apoptosis of HK-2 cells induced by CaOx crystals. We detected the optimal CaOx crystal concentration and intervention time by Western blot. ER stress modifiers tunicamycin (TM) and 4-phenylbutyric acid (4-PBA) were used to regulate the ER stress of HK-2 cells. The activities of ER stress marker proteins GRP78 and CHOP were evaluated by Western blot and immunohistochemistry. Western blot and TUNEL staining were used to detect cell apoptosis. We observed cell–crystal adhesion with an optical microscope. Lactate dehydrogenase (LDH) test kit and IL-1β enzyme-linked immunosorbent assay kit were used to detect and evaluate HK-2 cell damage. We found that the expression of ER stress marker proteins GRP78 and CHOP gradually increased with the increase in CaOx crystal concentration and intervention time and reached the maximum at 2.0 mmol/L and 24 h. The use of ER stress modifiers TM and 4-PBA can effectively regulate the ER stress level induced by CaOx crystals, and the level of apoptosis is positively correlated with the level of ER stress. 4-PBA pretreatment remarkably reduced cell–crystal adhesion and the secretions of IL-1β and LDH, whereas the results of TM pretreatment were the opposite. In summary, the damage and apoptosis of HK-2 cells induced by CaOx crystals are closely related to the level of ER stress. Inhibiting the ER stress of HK-2 cells can substantially reduce the cell damage and apoptosis induced by CaOx crystals.




Similar content being viewed by others
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Deng YL, Liu YL, Tao ZW, Wang X (2018) The role of cell-crystal reaction mediated inflammation in the formation of intrarenal calcium oxalate crystals. Zhonghua Wai Ke Za Zhi 56:733–736. https://doi.org/10.3760/cma.j.issn.0529-5815.2018.10.004
Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M (2005) Oxalate toxicity in renal cells. Urol Res 33:329–339. https://doi.org/10.1007/s00240-005-0485-3
Moryama MT, Domiki C, Miyazawa K et al (2005) Effects of oxalate exposure on Madin-Darby canine kidney cells in culture: renal prothrombin fragment-1 mRNA expression. Urol Res 33:470–475. https://doi.org/10.1007/s00240-005-0510-6
Khan SR, Shevock PN, Hackett RL (1992) Acute hyperoxaluria, renal injury and calcium oxalate urolithiasis. J Urol 147:226–230. https://doi.org/10.1016/s0022-5347(17)37202-6
Kang J, Sun Y, Deng Y et al (2020) Autophagy-endoplasmic reticulum stress inhibition mechanism of superoxide dismutase in the formation of calcium oxalate kidney stones. Biomed Pharmacother 121:109649. https://doi.org/10.1016/j.biopha.2019.109649
Sun Y, Liu Y, Guan X et al (2020) Atorvastatin inhibits renal inflammatory response induced by calcium oxalate crystals via inhibiting the activation of TLR4/NF-κB and NLRP3 inflammasome. IUBMB Life 72:1065–1074. https://doi.org/10.1002/iub.2250
Liu Y, Liu Q, Wang X et al (2018) Inhibition of autophagy attenuated ethylene glycol induced crystals deposition and renal injury in a rat model of nephrolithiasis. Kidney Blood Pressure Res 43:246–255. https://doi.org/10.1159/000487678
Yang B, Lu X, Li Y et al (2019) A proteomic network approach across the kidney stone disease reveals endoplasmic reticulum stress and crystal-cell interaction in the kidney. Oxid Med Cell Longev 2019:9307256. https://doi.org/10.1155/2019/9307256
Abhishek A, Benita S, Kumari M et al (2017) Molecular analysis of oxalate-induced endoplasmic reticulum stress mediated apoptosis in the pathogenesis of kidney stone disease. J Physiol Biochem 73:561–573. https://doi.org/10.1007/s13105-017-0587-8
Anelli T, Sitia R (2008) Protein quality control in the early secretory pathway. EMBO J 27:315–327. https://doi.org/10.1038/sj.emboj.7601974
Li H, Wen W, Xu H et al (2019) 4-Phenylbutyric acid protects against ethanol-induced damage in the developing mouse brain. Alcohol Clin Exp Res 43:69–78. https://doi.org/10.1111/acer.13918
Oakes SA, Papa FR (2015) The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 10:173–194. https://doi.org/10.1146/annurev-pathol-012513-104649
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664. https://doi.org/10.1172/JCI26373
Rasheva VI, Domingos PM (2009) Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14:996–1007. https://doi.org/10.1007/s10495-009-0341-y
Mimori S, Okuma Y, Kaneko M et al (2012) Protective effects of 4-phenylbutyrate derivatives on the neuronal cell death and endoplasmic reticulum stress. Biol Pharm Bull 35:84–90. https://doi.org/10.1248/bpb.35.84
Bhardwaj R, Tandon C, Dhawan DK, Kaur T (2017) Effect of endoplasmic reticulum stress inhibition on hyperoxaluria-induced oxidative stress: influence on cellular ROS sources. World J Urol 35:1955–1965. https://doi.org/10.1007/s00345-017-2083-8
Lieske JC, Huang E, Toback FG (2000) Regulation of renal epithelial cell affinity for calcium oxalate monohydrate crystals. Am J Physiol Renal Physiol 278:F130-137. https://doi.org/10.1152/ajprenal.2000.278.1.F130
Sun Y, Kang J, Tao Z et al (2020) Effect of endoplasmic reticulum stress-mediated excessive autophagy on apoptosis and formation of kidney stones. Life Sci 244:117232. https://doi.org/10.1016/j.lfs.2019.117232
Vivek K, Lourdes P de la V, Gerard F, C LJ (2005) Urinary macromolecular inhibition of crystal adhesion to renal epithelial cells is impaired in male stone formers. Kidney Int 68:1784–1792. https://doi.org/https://doi.org/10.1111/j.1523-1755.2005.00595.x
Randhawa R, Bhardwaj R, Kaur T (2019) Amelioration of hyperoxaluria-induced kidney dysfunction by chemical chaperone 4-phenylbutyric acid. Urolithiasis 47:171–179. https://doi.org/10.1007/s00240-018-1064-8
Khan SR, Hackett RL (1991) Retention of calcium oxalate crystals in renal tubules. Scanning Microsc 5:707–711; discussion 711–712
Bigelow MW, Wiessner JH, Kleinman JG, Mandel NS (1996) Calcium oxalate-crystal membrane interactions: dependence on membrane lipid composition. J Urol 155:1094–1098
Liu Q, Liu Y, Guan X, et al (2019) Effect of M2 Macrophages on Injury and Apoptosis of Renal Tubular Epithelial Cells Induced by Calcium Oxalate Crystals. Kidney Blood Press Res 1–15. https://doi.org/https://doi.org/10.1159/000501558
Zhu J, Wang Q, Li C et al (2019) Inhibiting inflammation and modulating oxidative stress in oxalate-induced nephrolithiasis with the Nrf2 activator dimethyl fumarate. Free Radic Biol Med 134:9–22. https://doi.org/10.1016/j.freeradbiomed.2018.12.033
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81960138 and 81760127), the Scientific Research and Technology Development Program of Guangxi (AB16380225) and the Natural Science Foundation of Guangxi Province (2018GXNSFBA138011, 2017GXNSFAA198158 and 2017GXNSFAA198070).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that he/she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The manuscript is approved by all authors for publication and no conflict of interest exists in the submission of this manuscript.
Rights and permissions
About this article
Cite this article
Sun, Y., Kang, J., Guan, X. et al. Regulation of endoplasmic reticulum stress on the damage and apoptosis of renal tubular epithelial cells induced by calcium oxalate crystals. Urolithiasis 49, 291–299 (2021). https://doi.org/10.1007/s00240-021-01261-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-021-01261-7