Abstract
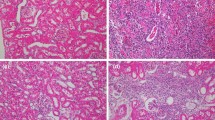
Despite its safety and efficacy, the traumatic effects of high-energy shock waves (HESW) on renal morphology and function during long-term follow-up have yet to be elucidated. Although the main target of shock waves is the stone located in the kidney, the surrounding tissue and other organs are also subjected to trauma during this procedure. In contrast to renal blood flow evaluation after shock wave treatment, ischemic development, causing varying degrees of damage at the tissue level, has not been well evaluated. . The renoprotective peptide adrenomedullin (AM) is a potent vasorelaxing, natriuretic and cell growth modulating peptide, which is thought to act as an autocrine/paracrine regulator in renal glomeruli and tubules. In this experimental study, renal parenchymal AM levels were assessed in an attempt to evaluate the effect of HESW on the tissue levels of this peptide, which may be responsible for the regulation of ischemia induced by extracorporeal shock wave lithotripsy(ESWL), in a rabbit model. Thirty white New Zealand rabbits, each weighing 3–5 kg were used. The animals were divided into three main groups, and varying numbers of shock waves (1,000, 1,500, 2,000) were applied under fluoroscopic localization to the same kidney of all animals. Ketamine HCl anesthesia was administered (15–20 mg/kg) and all of the procedures were performed with a Multimed 2000 lithotriptor. Untreated contralateral kidneys were evaluated as controls. Following HESW application, the treated and untreated kidneys of each animal were removed through bilateral flank incisions under ketamine HCl anesthesia after 24 h and 7 days, respectively. Tissue AM levels were assessed with immunohistochemistry. During the early follow-up period (24 h), both treated and untreated kidneys showed a moderate to high degree of AM positivity. The number of tubules stained with AM increased as the number of shock waves increased and the expression of this protein became evident, possibly due to a higher degree of tissue damage. Additionally, a limited degree of AM positivity was noted in the contralateral kidneys although this was not as evident as the positivity seen in the treated kidneys. Assessment of tissue AM levels during late follow-up (7 days) in both kidneys demonstrated a moderate or limited degree of positivity in the treated kidneys. Limited or no positivity could be demonstrated in the contralateral kidneys at this time.Taking the certain traumatic effects of HESW, which causes transient ischemia during ESWL, into account, we conclude that the application of HESW results in a transient decrease in renal perfusion, causing ischemic injury in treated as well as in contralateral (untreated) kidneys. This ischemic event lasts for a short time and seemed to be dose- and time-dependent. Increased tissue levels of AM appear to be a potential defence against ESWL induced ischemia.



Similar content being viewed by others
References
Akbay C, Sayın C, Sarıca K, Soygür T, Sabuncuoğlu B (1998) Effect of verapamil on rabbit renal tissue after shock wave lithotripsy: an ultrastructural approach. Electron Microsc 4: 493
Baltacı S, Özer G, Soygür T, Yaman Ö, Sarıca K, Müftüoğlu YZ, Göğüş O (1996) Effects of extracorporeal shock wave lithotripsy (ESWL) on urinary epidermal growth (EGF) levels. J Endourol 10: 519
Bauer V, Bauer F(1996) Reactive oxygen species as mediators of tissue protection and injury. Gen Physiol Biophys 18: 7
Bedük Y, Erden İ, Sarıca K, Aytaç S, Karalezli G, Göğüş O (1994) Evaluation of renal morphology and function by CFDS immediately after ESWL. J Endourol 7: 457
Bomanji J, Boddy S, Britton KE, Nimmon CC, Whitfield HN (1987) Radionuclide evaluation pre-and post extracorporeal shock wave lithotripsy for renal calculi. J Nucl Med 28: 1284
Frick J, Sarıca K, Köhle R, Kunit, G (1991) Long-term follow-up after extracorporeal shock wave lithotripsy in children. Eur Urol 19: 225
Hirata Y, Hayakawa H, Suzuki Y, Suzuki E, Ikenouchi H, Kohmoto O, Kimura K, Kitamura K, Eto T, Kangawa K, Matsuo H, Omata M (1995) Mechanisms of adrenomedullin-induced vasodilatation in the rat kidney. Hypertension 25: 790
Jougasaki M, Wei CM, Aarhus LL, Heublein DM, Sandberg SM, Burnett JC (1995) Renal localization and actions of adrenomedullin: a natriuretic peptide. Am J Physiol 268: F675
Karalezli G, Göğüş O, Bedük Y, Köküuslu C, Sarıca K, Köksal O (1993) Histopathologic effects of ESWL on rabbit kidney. Urol Res 21: 67
Kaude JV, Williams CM, Miliner MR, Scott KN, Finlayson B (1985) Renal morphology and function immediately after ESWL. Am J Roentgenol 145: 305
Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T (1995) Adrenomedullin: a novel hypotensive peptide isolated from human pheochromacytoma. Biochem Biophys Res Commun 192: 553
Krysiewicz S (1992) Complications of renal extracorporeal shock wave lithotripsy reviewed. Urol Radiol 13: 139
Küpeli B, Sarıca K, Koşar A, Alçığır G, Budak M, Özdiler E (1995) Histopathologic effects of high energy shock waves on rat ovary. In: Tiselius HG (ed) Renal stones: aspects on their formation, removal and prevention. Proceedings of the 6th European Symposium on Urolithiasis. Stockholm, Sweden, p 242
Mashiach E, Sela S, Winaver J, Shasha SM, Kristal B (1998) Renal ischemia-reperfusion injury: contribution of nitric oxide and renal blood flow. Nephron 80: 458
Mukoyama M, Sugavara A, Nagae T, Mori K, Murabe H, Itoh H, Tanaka I, Nakao K (2001) Role of adrenomedullin and its receptor system in renal pathophysiology. Peptides 22: 1925
Nagata D, Hirata Y, Suzuki E, Kakoki M, Hayaakawa H, Goto A, Ishimitsu T, Minamino N, Ono Y, Kangawa K, Matsuo H, Omata M (1999) Hypoxia induced adrenomedullin production in the kidney. Kidney Int 55: 1259
Sarıca K, Koşar A, Yaman O, Bedük Y, Durak I, Gögüş O, Kavukcu M (1996) Evaluation of ischemia after ESWL: detection of free oxygen radical scavenger enzymes in renal parenchyma subjected to high-energy shock waves. Urol Int 57 :221
Sarıca K, Soygür T, Yaman Ö., Özer G, Sayın N, Akbay C, Küpeli S, Yaman LS (1996) Stone recurrence after shock wave lithotripsy: evaluation of possible enhanced crystal deposition in traumatized tissue in rabbit model. J Endourol 10: 513
Sarıca K, Süzer O, Yaman O, Küpeli B, Baltaci S, Bilaloglu E, Tasman S (1996) Leucine aminopeptidase enzymuria: quantification of renal tubular damage following extracorporeal shock wave lithotripsy. Int Urol Nephrol 28: 621
Sarıca K, Özer G, Soygür T, Yaman Ö, Özer E, Ustün H, Yaman LS, Gogus O (1997) Preservation of shock wave-induced renal histologic changes by dermatan sulphate. Urology 49: 145
Sarıca K, Türkölmez K, Koşar A, Alçığır G, Özdiler E, Göğüş O (1998) Evaluation of basal membrane antibody (immunoglobulin G) formation after high energy shock wave application in rats. J Endourol 12: 505
Sarıca K, Bakır K, Yağcı F, Topçu O, Akbay C, Sayın N, Korkmaz C (1999) Limitation of possible enhanced crystal deposition by Verapamil in renal parenchyma after shock wave application in rabbit model. J Endourol 13: 343
Strohmaier WL, Lahme S, Weidenbach PM, Bichler KH (1999) Reduction of high-energy shock-wave-induced renal tubular injury by selenium. Urol Res 27: 382
Thamilselvan S, Byer KJ, Hackett RL, Khan SR (2000) Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol 64: 224
Willis LR, Evan AP, Connors BA, Blomgren P, Fineberg NS, Lingeman JE (1999) Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. J Am Soc Nephrol 10: 1753
Yaman Ö, Sarıca K, Özer G, Soygür T, Kutsal O, Yaman LS, Göğüş O (1996) Protective effect of Verepamil on renal histology following shock wave application in rabbit model. J Endourol.10: 329
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sarica, K., Sari, İ., Balat, A. et al. Evaluation of adrenomedullin levels in renal parenchyma subjected to extracorporeal shockwave lithotripsy. Urol Res 31, 267–271 (2003). https://doi.org/10.1007/s00240-003-0323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-003-0323-4