Abstract
Purpose
The assessment of multiple sclerosis (MS) lesions on follow-up magnetic resonance imaging (MRI) is tedious, time-consuming, and error-prone. Automation of low-level tasks could enhance the radiologist in this work. We evaluate the intelligent automation software Jazz in a blinded three centers study, for the assessment of new, slowly expanding, and contrast-enhancing MS lesions.
Methods
In three separate centers, 117 MS follow-up MRIs were blindly analyzed on fluid attenuated inversion recovery (FLAIR), pre- and post-gadolinium T1-weighted images using Jazz by 2 neuroradiologists in each center. The reading time was recorded. The ground truth was defined in a second reading by side-by-side comparison of both reports from Jazz and the standard clinical report. The number of described new, slowly expanding, and contrast-enhancing lesions described with Jazz was compared to the lesions described in the standard clinical report.
Results
A total of 96 new lesions from 41 patients and 162 slowly expanding lesions (SELs) from 61 patients were described in the ground truth reading. A significantly larger number of new lesions were described using Jazz compared to the standard clinical report (63 versus 24). No SELs were reported in the standard clinical report, while 95 SELs were reported on average using Jazz. A total of 4 new contrast-enhancing lesions were found in all reports. The reading with Jazz was very time efficient, taking on average 2min33s ± 1min0s per case. Overall inter-reader agreement for new lesions between the readers using Jazz was moderate for new lesions (Cohen kappa = 0.5) and slight for SELs (0.08).
Conclusion
The quality and the productivity of neuroradiological reading of MS follow-up MRI scans can be significantly improved using the dedicated software Jazz.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is an autoimmune, potentially disabling demyelinating disease of the central nervous system (CNS), with clinical onset typically in the young adult [1]. Traditionally, MS progression have been categorized in three main type, primary-progressive MS (PPMS) affecting between 10 and 15% of MS patients, relapsing–remitting MS (RRMS) and secondary-progressive MS (SPMS) [1], although it has been recently suggested that the clinical course of MS is better described as a continuum, with contributions from concurrent pathophysiological processes that vary across individuals and over time [2]. Prior to the availability of the disease-modifying therapies, roughly 50% of patients diagnosed with RRMS would transition to SPMS within 10 years, and 90% would transition within 25 years, with a median time of about 19 years [3]. The diagnosis of SPMS is most often established retrospectively [4], on average 3 years after the actual progression started [5]. This is caused by the difficulties by the clinicians and the patients to interpret the initial symptoms indicating early progression, which they can be subtle and fluctuating [4], and by the difficulties to detect disease progression on imaging. The importance of detecting remaining inflammatory disease earlier and more systematically has further increased with the demonstration that early intervention with high-efficacy disease modifying therapies can delay the onset of SPMS [6], and with the recent availability of approved new therapies for progressive MS, such as the anti-CD20 monoclonal antibody Ocrelizumab [7] or the selective sphingosine-1-phosphate receptor modulator Siponimod [8].
MS disease monitoring with imaging is particularly challenging. It involves at least yearly follow-up magnetic resonance imaging (MRI) scans of their central nervous system and consists of the tedious, time-consuming, and error-prone manual comparison and counting of the multiple demyelinating lesions. Given time constraints and the fact lesion burden can reach hundreds in severe cases, neuroradiologists can be forced to grossly compare the lesion load, and relevant disease evolution might remain unnoticed for some time. Longitudinal subtraction maps have been used to increase sensitivity for detecting new or enlarged lesions multiple sclerosis lesions [9,10,11,12] and reduce reading time [13], but small lesions might be difficult to detect using this technique [14].
Fully automated neuroradiological reporting of MS lesion load evolution would be of great interest, but current methods, including deep learning methods, have currently limited practicality given they are far from error free. In the last decade, deep learning methods, in particular two-dimensional and three-dimensional convolutional neural networks, have been applied to develop automatic lesion detection and segmentation [15]. Reported lesion-wise true positive rate ranges between 0.15 and 0.83, and the lesion-wise false positive rate ranges between 0.08 and 0.68 [16,17,18,19,20,21,22,23,24,25,26,27]. Those numbers are relatively low, given that every single new or evolving lesion on a follow-up control can be considered a marker of disease progression requiring a re-evaluation of the patient’s current therapy, and therefore, the expert radiologist assessment is still required for every patient [28].
In this context, a tool based on intelligent automation, which could simplify the work of the radiologist by automating many repetitive tasks, would be of interest. Intelligent automation aims to streamline processes to improve efficiency and reduce errors [29]. It applies the concept of breaking larger complex tasks into several simpler, very-well-understood low-level steps, and automatizes the low-level steps with intelligent software, using artificial intelligence and advanced software engineering. By freeing the radiologist to concentrate on the lesion assessment, it could reduce the number of missed lesions. Intelligent automation promises to improve productivity, reduce costs, as well as more precise processes and a better user experience. Jazz [30] is an intelligent automation software dedicated for neuroradiology, that automates multiple low-level tasks such as contrast recognition, previous exams organization, and images coregistration. It includes a semi-automatic lesion annotation tool, permitting the user to easily save all relevant information in a lesion list, and has a graphical user interface intended to maximize productivity and to minimize operator fatigue and discomfort (Fig. 1).
The intelligent automation software Jazz automizes low-level tasks for the radiologists, including (A) automatic contrast recognition, exams ordering, and (B) image coregistration for fast “single-click” lesion comparison and evaluation “without eye movements,” maximizing efficiency and minimizing errors. (C) Lesion locking, permits the evaluation of the lesion in various plane with a “single-click.” (D) Automatic anatomic localization and lesion tracking permits ultra-fast lesion navigation. The pre-populated drop-down pickers allow for fast corrections of lesion characteristics. From the lesion list, the software counts the lesions, summarizes the findings, and generates a report automatically, which permits an additional gain in time. Automated lesion counting and generation of a standardized, editable report, including overview figures of the lesions, saves additional time for the radiologist, and eliminates possible human errors in this step
In this retrospective study, we hypothesize that the quality and the productivity of neuroradiological reading of MS follow-up MRI scans can be improved using an intelligent automation software such as Jazz. We evaluated Jazz for the assessment of new, expanding, and contrast-enhancing MS lesions, blindly in three centers, and compared the reported lesions with the lesions described in the standard clinical report.
Methods
The following analysis was performed in three separate centers in a fully blinded and independent manner.
Dataset
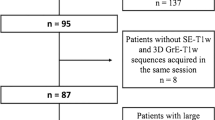
A set of 120 (40 from each of the 3 centers) current and prior magnetic resonance examinations of patients diagnosed with MS were obtained. 3D FLAIR, pre- and post-Gadolinium T1-weighted 3D sequences, as well as the corresponding clinical report, were analyzed. Images were acquired at 3 T (current exam 86%, previous exam 93%) and 1.5 T (current exam 14%, previous exam 7%), on machines from all three major vendors (GE, Siemens, and Philips). All data were anonymized.
Intelligent automation using Jazz
The intelligent automation software Jazz automizes low-level tasks for the radiologists as follows:
In a pre-processing step, Jazz automatically coregisters the images to one another using an affine transformation, label their contrast and organize the exams in chronological order. Single-click user interface tools permit lesion comparison and evaluation “without eye movements,” maximizing efficiency and minimizing errors. Lesion annotation occur with a single click, and include automatic anatomic localization detection. A pre-populated drop-down picker allow for fast corrections of lesion characteristics if necessary. A lesion list gets created and out of it, a report gets automatically generated, including lesion counting overview figures of the lesions, saves additional time for the radiologist, and eliminates possible human errors in this step (Fig. 1).
Case reading using Jazz
The cases were loaded in Jazz. The reader used then Jazz user interface to switch between the coregistered exams and contrasts using single-click operations or using the corresponding keyboard short-cut to evaluate the lesions. If necessary, the reader used the lesion locking tool to evaluate the lesion in different planes. When the user detected a new lesion, a slowly expanding lesion (SEL), or a contrast-enhancing lesion, the reader used Jazz lesion annotation tool, which permits marking of observed lesions with a single click, wherein the software automatically recognized the anatomical lesion location, and used if necessary the interactive lesion list with picker menus to correct the lesion description if necessary (Fig. 1).
In each center, two neuroradiologists evaluated independently all 40 cases using the Jazz software (center 1 CF, 12 years of experience, and NH, 8 years of experience, center 2, ME, 15 years of experience, and MM, 2 years of experience, center 3 NN, 5 years of experience, and JPU, 3 years of experience). The new lesions and the SELs were assessed on the 3D FLAIR. New lesions were defined as non-preexisting white matter lesions on the previous exam, visible on the current exam, while SELs were defined as preexisting white matter lesions that demonstrate lesions expansion between previous and current exam. The contrast-enhancing lesions were assessed by comparing the pre- and post-GAD T1-weighted 3D sequences. Reading time was recorded.
Ground truth
A ground truth was defined by reevaluating, using side-by-side comparison, all lesions described in the standard clinical reports and the reports generated by Jazz. The ground truth reading was done by either the most experienced reader of both readers (in two centers), or by a third experienced reader (third center, __Blinded for Review__, 8 years of experience). Based on this ground truth, the reported new lesions, SELs, and contrast-enhancing lesions in each report were then counted, and classified as true positive, false negative, or false positive.
Statistical analysis
Statistical significance was assessed using the Mann–Whitney U test between the average reading time with Jazz and generating the standard report. Significance level was set to α < 0.05. Inter-reader agreement between the readers using Jazz was assessed using Cohen’s Kappa.
Statement
This study was approved by the Ethic committee of the Université Paris, of the Canton of Zürich (2022–00041) and of Stanford University (IRB-26147). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent from the subjects was waived by the above-mentioned ethic committee. The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Results
Patient population
Thirty-nine cases were included from center 1 (mean age 55.5 ± 10.6 y, median age 57 y, age range 25–72 y, female / male = 30 / 9; one case was excluded because the lesions were very confluent, rendering the comparison of separate lesions difficult). Forty cases were included from center 2 (mean age 48.3 ± 12.8 y, median age 45 y, age range 26–73 y, female / male = 31 / 9). Thirty-eight cases were included from center 3 (mean age 35.4 ± 9.8 y, median age 34 y, age range 20–64 y, female / male = 20 / 18; one case was excluded because the follow-up MRI was done for hydrocephalus evaluation without dedicated description of the MS lesions, and one case was excluded because the 3D FLAIR was missing in one of the exams).
Reading time
Reading time using Jazz took on average 2 min 33 s ± 1 min 0 s per case for all readers (Fig. 2). In center 1, reading using Jazz took 2 min 29 s ± 2 min 15 s per case for reader 1, and 1 min 45 s ± 51 s for reader 2. In center 2, reading using Jazz took 4 min 30 s ± 2 min 42 s per case for reader 1, and 2 min 33 s ± 1 min 12 s for reader 2. In center 3, reading using Jazz took 2 min 12 s ± 1 min 7 s per case for reader 1, and 1 min 51 s ± 1 min 4 s for reader 2.
New lesions on 3D FLAIR
In all three centers, the ground truth reading reported an average of 0.83 ± 0.08 new lesions per case (a total of 96 lesions from 41 patients, out of a total of 117 patients). The standard clinical reports reported 24 true positive new lesions from 13 patients, 72 false negative, and 5 false positive new lesions. The Jazz report reported on average (average of 2 readers) 63 true positive lesions from 30.5 patients, 33 false negative, and 18 false positive new lesions (p < 0.05; Table 1 and Fig. 3).
Slowly expanding lesions on 3D FLAIR
In all three centers, the ground truth reading reported an average of 1.39 ± 0.72 SELs per case (a total of 162 lesions from 61 patients, out of a total of 117 patients). The standard clinical reports reported no growing lesions at any center. The Jazz reports reported on average (average of 2 readers) 95 true positive growing lesions from 45 patients, 67 false negative, and 38.5 false positive SELs (p < 0.05; Table 2 and Fig. 4).
Examples of SELs not described on the standard clinical report, but described by both readers with the Jazz software. Note that all those images were acquired from the same patient. While the presence of a single SEL might be often difficult to interpret, as it might occur from partial volume effects or from MRI artifacts, the presence of multiples SELs is highly suggestive of disease progression
Contrast-enhancing lesions
In center 1, all patients were administered intravenous contrast, and no contrast-enhancing lesions were described in any report. In center 2, 11 patients were not administered intravenous contrast; in the remaining 29 patients, 4 new contrast-enhancing lesions were described, consistently in all reports, and 1 false positive contrast-enhancing lesion was described on the standard clinical report only. In center 3, all patients except one were administered intravenous contrast; one contrast-enhancing lesion was described, consistently in all reports.
Inter-reader agreement
Overall inter-reader agreement for new lesions between the readers using Jazz was moderate, with kappa = 0.5 (center 1: 0.45, center 2: 0.51, center 3: 0.54) and for SELs it was slight, with kappa = 0.08 (center 1: -0.3, center 2: 0.3, center 3: 0.22).
Discussion
This study shows that a significantly larger number of new and SELs are reported when using the intelligent automation software Jazz compared to the standard clinical reporting method in a time efficient manner, by allowing the neuroradiologist to concentrate on the detection and evaluation of lesions. This is to our knowledge, the first study involving an intelligent automation software in the clinics. By permitting to toggle between coregistered current and previous exams, it uses a particularly potent neurophysiological mechanism to attract the attention of the reader to modifications in the images, in other words in the case of MS, to new and evolving lesions. Indeed, attention is a selective process and is physiologically necessary, because there are severe limits on our capacity to process visual information [31]. A theory suggests that attention is imposed by the fixed amount of overall energy available to the brain, and that because of the high-energy cost of neuronal activity, only a small fraction of the machinery can be engaged concurrently [31]. As a consequence, stimuli have to compete for limited resources [32], a notion that is supported by electrophysiological, neuroimaging and behavioral studies [33, 34]. Of the several visual stimuli that are known to capture attention, the sudden appearance of a new object and the sudden changes of an object are known to be particularly potent and to influence priority in visual search [35,36,37]. This mechanism is exploited in the dynamic switching tool of Jazz, compared to a standard side-by-side comparison method.
The identification of progression in MS is typically retrospective and based on clinical symptoms [38]. Most clinical trials rely on an increase in Expanded Disability Status Scale score confirmed over 3 or 6 months to define disease progression, and might overestimate the accumulation of permanent disability by up to 30% [39]. Given the profound burden of progressive MS and the recent development of effective treatments for these patients, there is a need to establish reliable and reproducible methods for identifying progression early in the disease course. Neuroradiological MRI reports of MS are often sparse and not standardized, despite the advantages of standardization of clinical data for MS monitoring being well recognized [40]. Structured reports of MRI in patients with MS have been shown to provide more adequate information for clinical decision making than nonstructured reports [41]. Improvement in the digitization of real-world patient data could foster new insights into the epidemiology and pathophysiology of the disease, and are ultimately necessary to achieve truthful personalized patient care [42].
This study also shows that at least one SEL was described in more than half of all patients, while none were reported in the standard clinical reports. The inter-reader agreement was low though, showing that this assessment remains particularly difficult and prone to subjectivity. The use of objective criteria defining SELs might help to improve this aspect. SELs can be easily overlooked, and are particularly difficult to detect in a standard DICOM viewer. There is substantial interest in SELs as a potential marker of chronic but active MS lesions [43,44,45] which may have diagnostic, prognostic and treatment implications. SELs were found to be more prevalent in patients with PPMS compared with patients with RRMS [44]. The proportion of SELs and their microstructural tissue abnormalities were associated with a higher risk of MS progression and SPMS conversion [46]. In SPMS, SELs were found to represent almost one-third of T2 lesions, associated with neurodegenerative MRI markers and related to clinical worsening [47]. Therefore, the number and volume of SELs are a promising biomarker to predict a more active, progressive disease course and could become a new target for therapeutic intervention. This is particularly interesting, because recent advances in our understanding of the mechanisms that drive SELs have fueled optimism for improved treatment of this condition [48]. A robust tool to gather SELs on magnetic resonance images, such as the one described in this work, might help to detect patients with progressive disease earlier and more systematically.
This study suffers from several limitations. Only three centers were included, and our results may not generalize to other centers. The reading using the Jazz software was not under identical conditions to the reading employed to generate the standard report, which was done during the standard clinical routine and might have included more distracting events. The reading of the standard clinical reports included at least a first reading and a second reading through a supervisor, and discussion with, and comments from the referring clinicians might have occurred. The reading with the Jazz software only included a single reading from a single reader. The ground truth was not based on a consensus reading performed by the most experienced reader in each center, which might have biased the agreement. Current and previous exams could have been acquired on different scanners, which might be a cofounder in the SEL evaluation. Further, a previous definition of SELs was based on the inclusion of a least three time points over more than 2 years [44], with local expansion assessed by the Jacobian determinant of the deformation between reference and follow-up scans, and a heuristic scored was used to favor individual SEL candidates undergoing concentric and constant change [44], while here, a more pragmatic approach was considered, using the subjective expansion assessment based on only two MR time points. Finally, no standardized method was used to define the SELs, as the evaluation was left to the subjective appraisal of each reader.
In conclusion, this study shows that significantly more new lesions and SELs can be described in a time-efficient manner using the intelligent automation software Jazz compared to the standard method.
Data availability
The data that support the findings of this study are available from the corresponding author, C.F., on reasonable request.
References
Thompson AJ, Baranzini SE, Geurts J et al (2018) Multiple sclerosis. Lancet 391:1622–1636. https://doi.org/10.1016/S0140-6736(18)30481-1
Kuhlmann T, Moccia M, Coetzee T et al (2023) Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol 22:78–88. https://doi.org/10.1016/S1474-4422(22)00289-7
Rovaris M, Confavreux C, Furlan R et al (2006) Secondary progressive multiple sclerosis: current knowledge and future challenges. Lancet Neurol 5:343–354. https://doi.org/10.1016/S1474-4422(06)70410-0
Klineova S, Lublin FD (2018) Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med 8:a028928. https://doi.org/10.1101/cshperspect.a028928
Katz Sand I, Krieger S, Farrell C, Miller AE (2014) Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler 20:1654–1657. https://doi.org/10.1177/1352458514521517
Brown JWL, Coles A, Horakova D et al (2019) Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 321:175. https://doi.org/10.1001/jama.2018.20588
Lamb YN (2022) Ocrelizumab: a review in multiple sclerosis. Drugs 82:323–334. https://doi.org/10.1007/s40265-022-01672-9
Kappos L, Bar-Or A, Cree BAC et al (2018) Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 391:1263–1273. https://doi.org/10.1016/S0140-6736(18)30475-6
Moraal B, Wattjes MP, Geurts JJG et al (2010) Improved detection of active multiple sclerosis lesions: 3D subtraction imaging. Radiology 255:154–163. https://doi.org/10.1148/radiol.09090814
Eichinger P, Wiestler H, Zhang H et al (2017) A novel imaging technique for better detecting new lesions in multiple sclerosis. J Neurol 264:1909–1918. https://doi.org/10.1007/s00415-017-8576-y
Eichinger P, Schön S, Pongratz V et al (2019) Accuracy of unenhanced MRI in the detection of new brain lesions in multiple sclerosis. Radiology 291:429–435. https://doi.org/10.1148/radiol.2019181568
Tan IL, Van Schijndel RA, Pouwels PJW et al (2002) Serial isotropic three-dimensional fast FLAIR imaging: using image registration and subtraction to reveal active multiple sclerosis lesions. Am J Roentgenol 179:777–782. https://doi.org/10.2214/ajr.179.3.1790777
Schmidt MA, Linker RA, Lang S et al (2018) FLAIRfusion processing with contrast inversion: improving detection and reading time of new cerebral MS lesions. Clin Neuroradiol 28:367–376. https://doi.org/10.1007/s00062-017-0567-y
Ganiler O, Oliver A, Diez Y et al (2014) A subtraction pipeline for automatic detection of new appearing multiple sclerosis lesions in longitudinal studies. Neuroradiology 56:363–374. https://doi.org/10.1007/s00234-014-1343-1
Afzal HMR, Luo S, Ramadan S, Lechner-Scott J (2022) The emerging role of artificial intelligence in multiple sclerosis imaging. Mult Scler 28:849–858. https://doi.org/10.1177/1352458520966298
Aslani S, Dayan M, Storelli L et al (2019) Multi-branch convolutional neural network for multiple sclerosis lesion segmentation. Neuroimage 196:1–15. https://doi.org/10.1016/j.neuroimage.2019.03.068
Hashemi SR, Salehi SSM, Erdogmus D et al (2019) Asymmetric loss functions and deep densely connected networks for highly imbalanced medical image segmentation: application to multiple sclerosis lesion detection. IEEE Access 7:1721–1735. https://doi.org/10.1109/ACCESS.2018.2886371
Andermatt S, Pezold S, Cattin PC (2018) Automated segmentation of multiple sclerosis lesions using multi-dimensional gated recurrent units. In: Crimi A, Bakas S, Kuijf H et al (eds) Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries. Springer International Publishing, Cham, pp 31–42
Valverde S, Cabezas M, Roura E et al (2017) Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage 155:159–168. https://doi.org/10.1016/j.neuroimage.2017.04.034
Maier O (2015) Handels H MS lesion segmentation in MRI with random forests. Proc Longitudinal Multiple Sclerosis Lesion Segmentation Challenge 1–2. https://iacl.ece.jhu.edu/images/d/d7/Oskar_Maier.pdf
Birenbaum A, Greenspan H (2016) Longitudinal multiple sclerosis lesion segmentation using multi-view convolutional neural networks. In: Carneiro G, Mateus D, Peter L et al (eds) Deep Learning and Data Labeling for Medical Applications. Springer International Publishing, Cham, pp 58–67
Deshpande H, Maurel P, Barillot C (2015) Adaptive dictionary learning for competitive classification of multiple sclerosis lesions. 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI). IEEE, Brooklyn, NY, USA, pp 136–139
Jain S, Sima DM, Ribbens A et al (2015) Automatic segmentation and volumetry of multiple sclerosis brain lesions from MR images. NeuroImage: Clin 8:367–375
Sudre CH, Cardoso MJ, Bouvy WH et al (2015) Bayesian model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Trans Med Imaging 34:2079–2102. https://doi.org/10.1109/TMI.2015.2419072
Tomas-Fernandez X, Warfield SK (2015) A model of population and subject (MOPS) intensities with application to multiple sclerosis lesion segmentation. IEEE Trans Med Imaging 34:1349–1361. https://doi.org/10.1109/TMI.2015.2393853
Hindsholm AM, Andersen FL, Cramer SP et al (2023) Scanner agnostic large-scale evaluation of MS lesion delineation tool for clinical MRI. Front Neurosci 17:1177540. https://doi.org/10.3389/fnins.2023.1177540
Krishnan AP, Song Z, Clayton D et al (2023) Multi-arm U-Net with dense input and skip connectivity for T2 lesion segmentation in clinical trials of multiple sclerosis. Sci Rep 13:4102. https://doi.org/10.1038/s41598-023-31207-5
Hainc N, Federau C, Stieltjes B et al (2017) The bright, artificial intelligence-augmented future of neuroimaging reading. Front Neurol 8:489. https://doi.org/10.3389/fneur.2017.00489
Wikipedia - Intelligent automation. https://en.wikipedia.org/wiki/Intelligent_automation. Accessed 1 Jan 2023
Jazz, www.ai-medical.ch
Carrasco M (2011) Visual attention: the past 25 years. Vision Res 51:1484–1525. https://doi.org/10.1016/j.visres.2011.04.012
Kinchla RA (1992) Attention. Annu Rev Psychol 43:711–742. https://doi.org/10.1146/annurev.ps.43.020192.003431
Beck DM, Kastner S (2009) Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Res 49:1154–1165. https://doi.org/10.1016/j.visres.2008.07.012
Reynolds JH, Chelazzi L (2004) Attentional modulation of visual processing. Annu Rev Neurosci 27:611–647. https://doi.org/10.1146/annurev.neuro.26.041002.131039
Enns JT, Austen EL, Lollo VD et al (2001) New objects dominate luminance transients in setting attentional priority. J Exp Psychol Hum Percept Perform 27(6):1287–1302
Yantis S, Hillstrom AP (1994) Stimulus-driven attentional capture: Evidence from equiluminant visual objects. J Exp Psychol Hum Percept Perform 20:95–107. https://doi.org/10.1037/0096-1523.20.1.95
Yantis S, Jonides J (1984) Abrupt visual onsets and selective attention: evidence from visual search. J Exp Psychol Hum Percept Perform 10:601–621. https://doi.org/10.1037/0096-1523.10.5.601
Filippi M, Preziosa P, Langdon D et al (2020) Identifying progression in multiple sclerosis: new perspectives. Ann Neurol 88:438–452. https://doi.org/10.1002/ana.25808
Kalincik T, Cutter G, Spelman T et al (2015) Defining reliable disability outcomes in multiple sclerosis. Brain 138:3287–3298. https://doi.org/10.1093/brain/awv258
D’Souza M, Papadopoulou A, Girardey C, Kappos L (2021) Standardization and digitization of clinical data in multiple sclerosis. Nat Rev Neurol 17:119–125. https://doi.org/10.1038/s41582-020-00448-7
Alessandrino F, Pichiecchio A, Mallucci G et al (2018) Do MRI structured reports for multiple sclerosis contain adequate information for clinical decision making? Am J Roentgenol 210:24–29. https://doi.org/10.2214/AJR.17.18451
Voigt I, Inojosa H, Dillenseger A et al (2021) Digital twins for multiple sclerosis. Front Immunol 12:669811. https://doi.org/10.3389/fimmu.2021.669811
Calvi A, Haider L, Prados F, et al (2020) In vivo imaging of chronic active lesions in multiple sclerosis. Mult Scler 135245852095858. https://doi.org/10.1177/1352458520958589
Elliott C, Wolinsky JS, Hauser SL et al (2019) Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler 25:1915–1925. https://doi.org/10.1177/1352458518814117
Elliott C, Belachew S, Wolinsky JS et al (2019) Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 142:2787–2799. https://doi.org/10.1093/brain/awz212
Preziosa P, Pagani E, Meani A et al (2022) Slowly expanding lesions predict 9-year multiple sclerosis disease progression. Neurol Neuroimmunol Neuroinflamm 9:e1139. https://doi.org/10.1212/NXI.0000000000001139
Calvi A, Carrasco FP, Tur C et al (2022) Association of slowly expanding lesions on MRI with disability in people with secondary progressive multiple sclerosis. Neurology 98:e1783–e1793. https://doi.org/10.1212/WNL.0000000000200144
Yong HYF, Yong VW (2022) Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis. Nat Rev Neurol 18:40–55. https://doi.org/10.1038/s41582-021-00581-x
Funding
Open access funding provided by University of Zurich This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
Christian Federau: study design, case reading, data analysis, manuscript writing; Nicolin Hainc: study design, case reading, manuscript editing Myriam Edjlali: study design, case reading, manuscript editing; Guangming Zhu: study design, data collection, manuscript editing; Milica Mastilovic: data collection, case reading, manuscript editing; Nathalie Nierobisch: case reading, manuscript editing; Jan-Philipp Uhlemann: case reading, manuscript editing; Silvio Paganucci: data analysis, manuscript analysis Cristina Granziera: study design, manuscript editing; Olivier Heinzlef: study design, manuscript editing; Lucas B. Kipp: study design, manuscript editing; Max Wintermark: study design, manuscript editing.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethic committee of the Université Paris, of the Canton of Zürich (2022–00041) and of Stanford University (IRB-26147).
Informed consent
Informed consent was waived.
Conflict of interest
The authors declare the following conflict of interest: CF: founder, employee and hold shares of AI Medical AG. SP: employee of AI Medical AG; LK: institution received research support from Genentech for MS clinical trials.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Federau, C., Hainc, N., Edjlali, M. et al. Evaluation of the quality and the productivity of neuroradiological reading of multiple sclerosis follow-up MRI scans using an intelligent automation software. Neuroradiology 66, 361–369 (2024). https://doi.org/10.1007/s00234-024-03293-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-024-03293-3