Abstract
Purpose
To compare resting-state functional connectivity (RSFC) of obese patients responders or non-responders to sleeve gastrectomy (SG) with a group of obese patients with no past medical history of metabolic or bariatric surgery.
Methods
MR images were acquired at 1.5 Tesla. Resting-state fMRI data were analyzed with statistical significance threshold set at p < 0.05, family-wise error (FWE) corrected.
Results
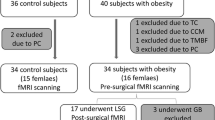
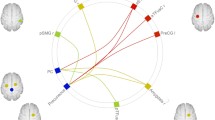
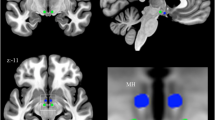
Sixty-two subjects were enrolled: 20 controls (age range 25–64; 14 females), 24 responders (excess weight loss > 50%; age range 23–68; 17 females), and 18 non-responders to sleeve gastrectomy (SG) (excess weight loss < 50%; age range 23–67; 13 females). About within-network RSFC, responders showed significantly lower RSFC with respect to both controls and non-responders in the default mode and frontoparietal networks, positively correlating with psychological scores. Non-responders showed significantly higher (p < 0.05, family-wise error (few) corrected) RSFC in regions of the lateral visual network as compared to controls. Regarding between-network RSFC, responders showed significantly higher anti-correlation between executive control and salience networks (p < 0.05, FWE corrected) with respect to both controls and non-responders. Significant positive correlation (Spearman rho = 0.48, p = 0.0012) was found between % of excess weight loss and executive control-salience network RSFC.
Conclusion
There are differences in brain functional connectivity in either responders or non-responders patients to SG. The present results offer new insights into the neural correlates of outcome in patients who undergo SG and expand knowledge about neural mechanisms which may be related to surgical response.




Similar content being viewed by others
Data availability
Data are available upon reasonable requests of collaboration.
Code availability
None.
Abbreviations
- RSFC:
-
Resting-state functional connectivity
- SG:
-
Sleeve gastrectomy
- FWE:
-
Family-wise error
- EWL:
-
Excess weight loss
- fMRI:
-
Functional MRI
- BOLD:
-
Blood oxygen level-dependent
- FC:
-
Functional connectivity
- TIV:
-
Total intracranial volume
- TFCE:
-
Threshold-free cluster enhancement
- %EWL:
-
Percentage of excess weight loss
- BES:
-
Binge Eating Scale
- SOM:
-
Somatization
- OBS\COMP:
-
Obsessive-compulsive
- IS:
-
Interpersonal sensitivity
- PAR:
-
Paranoid thought
- GSI:
-
General Symptom Index
- RSNs:
-
Resting-state networks
- ICA:
-
Independent component analysis
References
Hedley AA, Ogden CL, Johnson CL, et al (2004) Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. J Am Med Assoc 291: https://doi.org/10.1001/jama.291.23.2847
Phillips BT, Shikora SA (2018) The history of metabolic and bariatric surgery: development of standards for patient safety and efficacy. Metabolism 79:97–107. https://doi.org/10.1016/j.metabol.2017.12.010
Hill JO (1979) Peters JC (1998) Environmental contributions to the obesity epidemic. Science 280:1371–1374. https://doi.org/10.1126/science.280.5368.1371
Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003641.pub4
Nguyen N, Champion JK, Ponce J et al (2012) A review of unmet needs in obesity management. Obes Surg 22:956–966. https://doi.org/10.1007/s11695-012-0634-z
Golomb I, ben David M, Glass A, et al (2015) Long-term metabolic effects of laparoscopic sleeve gastrectomy. JAMA Surg 150:1051. https://doi.org/10.1001/jamasurg.2015.2202
Smith SM, Vidaurre D, Beckmann CF et al (2013) Functional connectomics from resting-state fMRI. Trends Cogn Sci 17:666–682. https://doi.org/10.1016/j.tics.2013.09.016
Mallio C, Zobel B, Quattrocchi C (2015) Evaluating rehabilitation interventions in Parkinson’s disease with functional MRI: a promising neuroprotective strategy. Neural Regen Res 10:702. https://doi.org/10.4103/1673-5374.156957
Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med 34:537–541. https://doi.org/10.1002/mrm.1910340409
Mallio CA, Piervincenzi C, Gianolio E et al (2019) Absence of dentate nucleus resting-state functional connectivity changes in nonneurological patients with gadolinium-related hyperintensity on T1-weighted images. J Magn Reson Imaging 50:445–455. https://doi.org/10.1002/jmri.26669
Quattrocchi CC, de Pandis MF, Piervincenzi C et al (2015) Acute modulation of brain connectivity in Parkinson disease after automatic mechanical peripheral stimulation: a pilot study. PLoS ONE 10:e0137977. https://doi.org/10.1371/journal.pone.0137977
Mallio CA, Piervincenzi C, Carducci F et al (2020) Within-network brain connectivity in Crohn’s disease patients with gadolinium deposition in the cerebellum. Neuroradiology 62:833–841. https://doi.org/10.1007/s00234-020-02415-x
Legget KT, Wylie KP, Cornier M-A et al (2021) Altered between-network connectivity in individuals prone to obesity. Physiol Behav 229:113242. https://doi.org/10.1016/j.physbeh.2020.113242
Moreno-Lopez L, Contreras-Rodriguez O, Soriano-Mas C et al (2016) Disrupted functional connectivity in adolescent obesity. NeuroImage Clinical 12:262–268. https://doi.org/10.1016/j.nicl.2016.07.005
Park B, Byeon K, Lee MJ et al (2020) Whole-brain functional connectivity correlates of obesity phenotypes. Hum Brain Mapp 41:4912–4924. https://doi.org/10.1002/hbm.25167
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. https://doi.org/10.1016/0028-3932(71)90067-4
Derogatis LR, Unger R (2010) Symptom checklist-90-revised. In: The Corsini Encyclopedia of Psychology. John Wiley & Sons, Inc., Hoboken. https://doi.org/10.1002/9780470479216.corpsy0970
Fossati A, di Ceglie A, Acquarini E, Barratt ES (2001) Psychometric properties of an Italian version of the Barratt Impulsiveness Scale-11 (BIS-11) in nonclinical subjects. J Clin Psychol 57:815–828. https://doi.org/10.1002/jclp.1051
Sighinolfi C, Norcini Pala A, Chiri LR et al (2010) Difficulties in emotion regulation scale (DERS): the Italian translation and adaptation. Psicoter Cogn Comport 16:141–170
di Bernardo M, Barciulli E, Ricca V et al (1998) Binge Eating Scale in obese patients: validation of the Italian version. Minerva Psichiatr 39:125–130
Esteban O, Markiewicz CJ, Blair RW et al (2019) fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16:111–116. https://doi.org/10.1038/s41592-018-0235-4
Gorgolewski K, Burns CD, Madison C, et al (2011) Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. Frontiers in Neuroinformatics 5: https://doi.org/10.3389/fninf.2011.00013
Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005) Investigations into resting-state connectivity using independent component analysis. Philosl Trans R Soc B Biol Sci 360:1001–1013. https://doi.org/10.1098/rstb.2005.1634
Smith S, Nichols T (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061
Smith SM, Fox PT, Miller KL et al (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci 106:13040–13045. https://doi.org/10.1073/pnas.0905267106
Thomas Yeo BT, Krienen FM, Sepulcre J et al (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. https://doi.org/10.1152/jn.00338.2011
Nickerson LD, Smith SM, Öngür D, Beckmann CF (2017) Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Frontiers in Neuroscience 11: https://doi.org/10.3389/fnins.2017.00115
Filippini N, MacIntosh BJ, Hough MG et al (2009) Distinct patterns of brain activity in young carriers of the APOE -ε4 allele. Proc Natl Acad Sci 106:7209–7214. https://doi.org/10.1073/pnas.0811879106
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25. https://doi.org/10.1002/hbm.1058
Hayasaka S, Nichols TE (2004) Combining voxel intensity and cluster extent with permutation test framework. Neuroimage 23:54–63. https://doi.org/10.1016/j.neuroimage.2004.04.035
Seeley WW, Menon V, Schatzberg AF et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Seeley WW (2019) The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci 39:9878–9882. https://doi.org/10.1523/JNEUROSCI.1138-17.2019
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network. Ann N Y Acad Sci 1124:1–38. https://doi.org/10.1196/annals.1440.011
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci 100:253–258. https://doi.org/10.1073/pnas.0135058100
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447. https://doi.org/10.1146/annurev-neuro-071013-014030
Utevsky Av, Smith Dv, Huettel SA (2014) Precuneus is a functional core of the default-mode networK. J Neurosci 34:932–940. https://doi.org/10.1523/JNEUROSCI.4227-13.2014
Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. https://doi.org/10.1038/35094500
Eustache F, Piolino P, Giffard B et al (2004) “In the course of time”: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain 127:1549–1560. https://doi.org/10.1093/brain/awh166
Lundstrom BN, Ingvar M, Petersson KM (2005) The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 27:824–834. https://doi.org/10.1016/j.neuroimage.2005.05.008
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583. https://doi.org/10.1093/brain/awl004
Marek S, Dosenbach NUF (2018) The frontoparietal network: function electrophysiology and importance of individual precision mapping. Dialogues in Clinical Neuroscience 20:133–140. https://doi.org/10.31887/DCNS.2018.20.2/smarek
Rehman A, al Khalili Y, (2021) Neuroanatomy, Occipital Lobe. StatPearls Publishing, StatPearls
Elton A, Gao W (2014) Divergent task-dependent functional connectivity of executive control and salience networks. Cortex 51:56–66. https://doi.org/10.1016/j.cortex.2013.10.012
García-García I, Jurado MÁ, Garolera M et al (2013) Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp 34:2786–2797. https://doi.org/10.1002/hbm.22104
Borowitz MA, Yokum S, Duval ER, Gearhardt AN (2020) Weight-related differences in salience, default mode, and executive function network connectivity in adolescents. Obesity 28:1438–1446. https://doi.org/10.1002/oby.22853
Weissman DH (2004) The neural mechanisms for minimizing cross-modal distraction. J Neurosci 24:10941–10949. https://doi.org/10.1523/JNEUROSCI.3669-04.2004
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215. https://doi.org/10.1038/nrn755
Cerit H, Davidson P, Hye T et al (2019) Resting-state brain connectivity predicts weight loss and cognitive control of eating behavior after vertical sleeve gastrectomy. Obesity 27:1846–1855. https://doi.org/10.1002/oby.22607
Wang Y, Ji G, Hu Y et al (2020) Laparoscopic sleeve gastrectomy induces sustained changes in gray and white matter brain volumes and resting functional connectivity in obese patients. Surg Obe Relat Dis 16:1–9. https://doi.org/10.1016/j.soard.2019.09.074
Schwartz MB, Brownell KD (2004) Obesity and body image. Body Image 1:43–56. https://doi.org/10.1016/S1740-1445(03)00007-X
Lasaponara S, Mauro F, Carducci F, et al (2017) Increased alpha band functional connectivity following the quadrato motor training: a longitudinal study. Frontiers in Human Neuroscience 11: https://doi.org/10.3389/fnhum.2017.00282
Quattrocchi CC, Giona A, di Martino A et al (2015) Lumbar subcutaneous edema and degenerative spinal disease in patients with low back pain: a retrospective MRI study. Musculoskelet Surg 99:159–163. https://doi.org/10.1007/s12306-015-0355-2
Greco F, Quarta LG, Grasso RF et al (2020) Increased visceral adipose tissue in clear cell renal cell carcinoma with and without peritumoral collateral vessels. Br J Radiol 93:20200334. https://doi.org/10.1259/bjr.20200334
Greco F, Mallio CA, Grippo R et al (2020) Increased visceral adipose tissue in male patients with non-clear cell renal cell carcinoma. Radiol Med (Torino) 125:538–543. https://doi.org/10.1007/s11547-020-01146-6
Sima E, Webb D-L, Hellström PM, Sundbom M (2019) Non-responders after gastric bypass surgery for morbid obesity: peptide hormones and glucose homeostasis. Obes Surg 29:4008–4017. https://doi.org/10.1007/s11695-019-04089-8
Cassidy RM, Tong Q (2017) Hunger and satiety gauge reward sensitivity. Frontiers in Endocrinology 8: s10.3389/fendo.2017.00104
Banks WA, Tschöp M, Robinson SM, Heiman ML (2002) Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302:822–827. https://doi.org/10.1124/jpet.102.034827
Schmidt L, Medawar E, Aron-Wisnewsky J, et al (2021) Resting-state connectivity within the brain’s reward system predicts weight loss and correlates with leptin. Brain Communications 3: https://doi.org/10.1093/braincomms/fcab005
Funding
None.
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to all the categories established by the International Committee of Medical Journal Editors (ICMJE) guidelines on authorship:
1. Carlo Augusto Mallio: conception and design, acquisition of data, analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
2. Giuseppe Spagnolo: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
3. Claudia Piervincenzi: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
4. Nikolaos Petsas: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
5. Danilo Boccetti: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
6. Federica Spani: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
7. Ida Francesca Gallo: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
8. Antonella Sisto: acquisition of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
9. Livia Quintiliani: acquisition of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
10. Gianfranco Di Gennaro: acquisition of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
11. Vincenzo Bruni: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
12. Carlo Cosimo Quattrocchi: conception and design, acquisition of data, analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflicts of interest
All the authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the Ethical Committee of our institution (30/20 PAR ComEt CBM) and was designed as prospective case–control.
Consent to participate
The consent to participate was obtained from all the subjects.
Consent for publication
The consent for publication was obtained from all the subjects.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mallio, C.A., Spagnolo, G., Piervincenzi, C. et al. Brain functional connectivity differences between responders and non-responders to sleeve gastrectomy. Neuroradiology 65, 131–143 (2023). https://doi.org/10.1007/s00234-022-03043-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03043-3