Abstract
Purpose
The hypothalamus plays a pivotal role in the pathogenesis of narcolepsy. This study aimed to evaluate the differences in the structural covariance network of thehypothalamus based on volume differences between patients with narcolepsy and healthy controls.
Methods
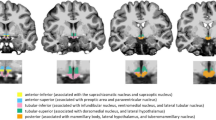
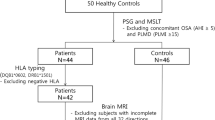
We retrospectively enrolled 15 patients with narcolepsy and 19 healthy controls.All subjects underwent three-dimensional T1-weighted imaging using a 3-T magnetic resonance imaging scanner. Hypothalamic subunits were segmented, and the volumes of individual hypothalamic subunits were obtained using the FreeSurfer program. Subsequently, we conducted a structural covariance network analysis of the subunit volumes with graph theory using the BRAPH program in patients with narcolepsy and in healthy controls.
Results
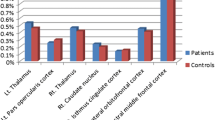
There were no significant differences in the volumes of the entire right and left hypothalamus nor in the hypothalamic subunit between patients with narcolepsy and healthy controls. However, we found significant differences in the structural covariance network in the hypothalamus between these groups. The characteristic path length was significantly lower in patients with narcolepsy than in healthy controls (1.698 vs. 2.831, p = 0.001). However, other network measures did not differ between patients with narcolepsy and healthy controls.
Conclusion
We found that the structural covariance network of the hypothalamus, as assessed from the subunit volumes of hypothalamic regions using a graph theoretical analysis, is different in patients with narcolepsy compared to healthy controls. These findings may contribute to the understanding of the pathogenesis of narcolepsy.

Similar content being viewed by others
Data availability
Data that support the findings of this study are available upon reasonable request.
Code availability
Not applicable.
References
Anderson D (2021) Narcolepsy: a clinical review. JAAPA 34(6):20–25. https://doi.org/10.1097/01.JAA.0000750944.46705.36
Bassetti CLA, Adamantidis A, Burdakov D, Han F, Gay S, Kallweit U, Khatami R, Koning F, Kornum BR, Lammers GJ, Liblau RS, Luppi PH, Mayer G, Pollmacher T, Sakurai T, Sallusto F, Scammell TE, Tafti M, Dauvilliers Y (2019) Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol 15(9):519–539. https://doi.org/10.1038/s41582-019-0226-9
Ruoff C, Rye D (2016) The ICSD-3 and DSM-5 guidelines for diagnosing narcolepsy: clinical relevance and practicality. Curr Med Res Opin 32(10):1611–1622. https://doi.org/10.1080/03007995.2016.1208643
Mahoney CE, Cogswell A, Koralnik IJ, Scammell TE (2019) The neurobiological basis of narcolepsy. Nat Rev Neurosci 20(2):83–93. https://doi.org/10.1038/s41583-018-0097-x
Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437(7063):1257–1263. https://doi.org/10.1038/nature04284
Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM (2000) Reduced number of hypocretin neurons in human narcolepsy. Neuron 27(3):469–474. https://doi.org/10.1016/s0896-6273(00)00058-1
Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S (2002) The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 59(10):1553–1562. https://doi.org/10.1001/archneur.59.10.1553
Ballotta D, Talami F, Pizza F, Vaudano AE, Benuzzi F, Plazzi G, Meletti S (2021) Hypothalamus and amygdala functional connectivity at rest in narcolepsy type 1. Neuroimage Clin 31:102748. https://doi.org/10.1016/j.nicl.2021.102748
Schwartz S, Ponz A, Poryazova R, Werth E, Boesiger P, Khatami R, Bassetti CL (2008) Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain 131(Pt 2):514–522. https://doi.org/10.1093/brain/awm292
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95(1):322–327. https://doi.org/10.1073/pnas.95.1.322
Brabec J, Rulseh A, Horinek D, Pala A, Guerreiro H, Buskova J, Petrovicky P, Nemcova V, Krasensky J, Seidl Z, Nimsky C, Sonka K (2011) Volume of the amygdala is reduced in patients with narcolepsy - a structural MRI study. Neuro Endocrinol Lett 32(5):652–656
Draganski B, Geisler P, Hajak G, Schuierer G, Bogdahn U, Winkler J, May A (2002) Hypothalamic gray matter changes in narcoleptic patients. Nat Med 8(11):1186–1188. https://doi.org/10.1038/nm1102-1186
Overeem S, Steens SC, Good CD, Ferrari MD, Mignot E, Frackowiak RS, van Buchem MA, Lammers GJ (2003) Voxel-based morphometry in hypocretin-deficient narcolepsy. Sleep 26(1):44–46
Buskova J, Vaneckova M, Sonka K, Seidl Z, Nevsimalova S (2006) Reduced hypothalamic gray matter in narcolepsy with cataplexy. Neuro Endocrinol Lett 27(6):769–772
Kaufmann C, Schuld A, Pollmacher T, Auer DP (2002) Reduced cortical gray matter in narcolepsy: preliminary findings with voxel-based morphometry. Neurology 58(12):1852–1855. https://doi.org/10.1212/wnl.58.12.1852
Hofman MA, Swaab DF (1992) The human hypothalamus: comparative morphometry and photoperiodic influences. Prog Brain Res 93:133–147; discussion 148–139. https://doi.org/10.1016/s0079-6123(08)64569-0
Billot B, Bocchetta M, Todd E, Dalca AV, Rohrer JD, Iglesias JE (2020) Automated segmentation of the hypothalamus and associated subunits in brain MRI. Neuroimage 223:117287. https://doi.org/10.1016/j.neuroimage.2020.117287
Guo C, Ferreira D, Fink K, Westman E, Granberg T (2019) Repeatability and reproducibility of FreeSurfer, FSL-SIENAX and SPM brain volumetric measurements and the effect of lesion filling in multiple sclerosis. Eur Radiol 29(3):1355–1364. https://doi.org/10.1007/s00330-018-5710-x
Lee DA, Lee HJ, Kim HC, Park KM (2021) Alterations of the structural covariance network in the hypothalamus of patients with cluster headache. J Neurol. https://doi.org/10.1007/s00415-021-10629-z
Mijalkov M, Kakaei E, Pereira JB, Westman E, Volpe G, Alzheimer’s Disease Neuroimaging I (2017) BRAPH: a graph theory software for the analysis of brain connectivity. PLoS One 12 (8):e0178798. https://doi.org/10.1371/journal.pone.0178798
Farahani FV, Karwowski W, Lighthall NR (2019) Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Front Neurosci 13:585. https://doi.org/10.3389/fnins.2019.00585
Welton T, Kent DA, Auer DP, Dineen RA (2015) Reproducibility of graph-theoretic brain network metrics: a systematic review. Brain Connect 5(4):193–202. https://doi.org/10.1089/brain.2014.0313
Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N (2011) Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 21(9):2147–2157. https://doi.org/10.1093/cercor/bhq291
Rossini PM, Di Iorio R, Bentivoglio M, Bertini G, Ferreri F, Gerloff C, Ilmoniemi RJ, Miraglia F, Nitsche MA, Pestilli F, Rosanova M, Shirota Y, Tesoriero C, Ugawa Y, Vecchio F, Ziemann U, Hallett M (2019) Methods for analysis of brain connectivity: An IFCN-sponsored review. Clin Neurophysiol 130(10):1833–1858. https://doi.org/10.1016/j.clinph.2019.06.006
Mite Mijalkov EK, Joana B. Pereira, Eric Westman, Giovanni Volpe (2016) BRAPH 1.0.0 Manual. BRAPH.ORG. http://braph.org/wpdm-package/braph-1-0-0/.
John J, Thannickal TC, McGregor R, Ramanathan L, Ohtsu H, Nishino S, Sakai N, Yamanaka A, Stone C, Cornford M, Siegel JM (2013) Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann Neurol 74(6):786–793. https://doi.org/10.1002/ana.23968
Valko PO, Gavrilov YV, Yamamoto M, Reddy H, Haybaeck J, Mignot E, Baumann CR, Scammell TE (2013) Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol 74(6):794–804. https://doi.org/10.1002/ana.24019
Thannickal TC, Siegel JM, Nienhuis R, Moore RY (2003) Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol 13(3):340–351. https://doi.org/10.1111/j.1750-3639.2003.tb00033.x
Nishino S, Sakurai E, Nevsimalova S, Yoshida Y, Watanabe T, Yanai K, Mignot E (2009) Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep 32(2):175–180. https://doi.org/10.1093/sleep/32.2.175
Dauvilliers Y, Delallee N, Jaussent I, Scholz S, Bayard S, Croyal M, Schwartz JC, Robert P (2012) Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions. Sleep 35(10):1359–1366. https://doi.org/10.5665/sleep.2114
John J, Wu MF, Boehmer LN, Siegel JM (2004) Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron 42(4):619–634. https://doi.org/10.1016/s0896-6273(04)00247-8
Lopez R, Barateau L, Evangelista E, Chenini S, Robert P, Jaussent I, Dauvilliers Y (2017) Temporal changes in the cerebrospinal fluid level of hypocretin-1 and histamine in narcolepsy. Sleep 40 (1). https://doi.org/10.1093/sleep/zsw010
Hublin C, Partinen M, Kaprio J, Koskenvuo M, Guilleminault C (1994) Epidemiology of narcolepsy. Sleep 17(8 Suppl):S7-12. https://doi.org/10.1093/sleep/17.suppl_8.s7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, H.C., Lee, D.A., Lee, HJ. et al. Alterations in the structural covariance network of the hypothalamus in patients with narcolepsy. Neuroradiology 64, 1351–1357 (2022). https://doi.org/10.1007/s00234-021-02878-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02878-6