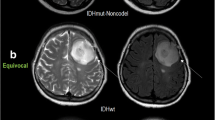
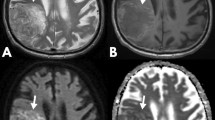
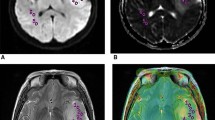
Abstract
Purpose
H3K27M-mutant diffuse midline gliomas (M-DMGs) exhibit a clinically aggressive course. We studied diffusion-weighted imaging (DWI) and perfusion (PWI) MRI features of DMG with the hypothesis that DWI-PWI metrics can serve as biomarkers for the prediction of the H3K27M mutation status in DMGs.
Methods
A retrospective review of the institutional database (imaging and histopathology) of patients with DMG (July 2016 to July 2020) was performed. Tumoral apparent diffusion coefficient (ADC) and peritumoral ADC (PT ADC) values and their normalized values (nADC and nPT ADC) were computed. Perfusion data were analyzed with manual arterial input function (AIF) and leakage correction (LC) Boxerman-Weiskoff models. Normalized maximum relative CBV (rCBV) was evaluated. Intergroup analysis of the imaging variables was done between M-DMGs and wild-type (WT-DMGs) groups.
Results
Ninety-four cases (M-DMGs-n = 48 (51%) and WT-DMGs-n = 46(49%)) were included. Significantly lower PT ADC (mutant—1.1 ± 0.33, WT—1.23 ± 0.34; P = 0.033) and nPT ADC (mutant—1.64 ± 0.48, WT—1.83 ± 0.54; P = 0.040) were noted in the M-DMGs. The rCBV (mutant—25.17 ± 27.76, WT—13.73 ± 14.83; P = 0.018) and nrCBV (mutant—3.44 ± 2.16, WT—2.39 ± 1.25; P = 0.049) were significantly higher in the M-DMGs group. Among thalamic DMGs, the min ADC, PT ADC, and nADC and nPT ADC were lower in M-DMGs while nrCBV (corrected and uncorrected) was significantly higher. Receiver operator characteristic curve analysis demonstrated that PT ADC (cut-off—1.245), nPT ADC (cut-off—1.853), and nrCBV (cut-off—1.83) were significant independent predictors of H3K27M mutational status in DMGs.
Conclusion
DWI and PWI features hold value in preoperative prediction of H3K27M-mutation status in DMGs.






Similar content being viewed by others
References
Alelú-Paz R, Ashour N, González-Corpas A, Ropero S (2012) DNA methylation, histone modifications, and signal transduction pathways: a close relationship in malignant gliomas pathophysiology. J Signal Transduct 2012:1–8
Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E et al (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 124(3):439–47
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–31. https://doi.org/10.1038/nature10833
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB et al (2018) cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 135(4):639–42. https://doi.org/10.1007/s00401-018-1826-y
Daoud EV, Rajaram V, Cai C, Oberle RJ, Martin GR, Raisanen JM et al (2018) Adult brainstem gliomas with H3K27M mutation: radiology, pathology, and prognosis. J Neuropathol Exp Neurol 77(4):302–311
Johnson DR, Guerin JB, Giannini C, Morris JM, Eckel LJ, Kaufmann TJ (2017) 2016 updates to the WHO brain tumor classification system: what the radiologist needs to know. Radiographics 37(7):2164–2180
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, et al. The 2016 WHO classification of tumors of the central nervous system. International Agency for Research on Cancer (IARC), Lyon. 2016.
Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJJ et al (2016) Diffuse midline gliomas with histone H3–K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol 26(5):569–580
Lu QR, Qian L, Zhou X (2019) Developmental origins and oncogenic pathways in malignant brain tumors. Wiley Interdiscip Rev Dev Biol 8(4):1–23
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251
Gojo J, Pavelka Z, Zapletalova D, Schmook MT, Mayr L, Madlener S et al (2020) Personalized treatment of H3K27M-mutant pediatric diffuse gliomas provides improved therapeutic opportunities. Front Oncol 9(January):1–14
Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M et al (2018) Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol 20(1):123–131
Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C (2014) Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 128(4):573–581
Chen H, Hu W, He H, Yang Y, Wen G, Lv X (2019) Noninvasive assessment of H3 K27M mutational status in diffuse midline gliomas by using apparent diffusion coefficient measurements. Eur J Radiol 114:152–159
Schreck KC, Ranjan S, Skorupan N, Bettegowda C, Eberhart CG, Ames HM et al (2019) Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol. 143(1):87–93. https://doi.org/10.1007/s11060-019-03134-x
Aboian MS, Solomon DA, Felton E, Mabray MC, Villanueva-Meyer JE, Mueller S et al (2017) Imaging characteristics of pediatric diffuse midline gliomas with histone H3 K27M mutation. Am J Neuroradiol 38(4):795–800
Thust SC, Hassanein S, Bisdas S, Rees JH, Hyare H, Maynard JA et al (2018) Apparent diffusion coefficient for molecular subtyping of non-gadolinium-enhancing WHO grade II/III glioma: volumetric segmentation versus two-dimensional region of interest analysis. Eur Radiol 28:3779–3788
Lee S, Choi SH, Ryoo I, Yoon TJ, Kim TM, Lee SH et al (2015) Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J Neurooncol 121(1):141–150
Patterson DM, Padhani AR, Collins DJ (2008) Technology insight: water diffusion MRI - a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 5:220–233
Cui Y, Ma L, Chen X, Zhang Z, Jiang H, Lin S (2014) Lower apparent diffusion coefficients indicate distinct prognosis in low-grade and high-grade glioma. J Neurooncol 119:377–385
Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML et al (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247(2):490–498
Rossi A, Gandolfo C, Morana G, Severino M, Garrè ML, Cama A (2010) New MR sequences (diffusion, perfusion, spectroscopy) in brain tumours. Pediatr Radiol 40(6):999–1009
Xing Z, Yang X, She D, Lin Y, Zhang Y, Cao D (2017) Noninvasive assessment of IDH mutational status in World Health Organization grade II and III astrocytomas using DWI and DSC-PWI combined with conventional MR imaging. Am J Neuroradiol 38(6):1138–1144
Yamashita K, Hiwatashi A, Togao O, Kikuchi K, Hatae R, Yoshimoto K et al (2016) MR imaging-based analysis of glioblastoma multiforme: estimation of IDH1 mutation status. Am J Neuroradiol 37(1):58–65
Darbar A, Waqas M, Enam SF, Mahmood SD (2018) Use of preoperative apparent diffusion coefficients to predict brain tumor grade. Cureus. 10(3):e2284
Murakami R, Hirai T, Sugahara T, Fukuoka H, Toya R, Nishimura S et al (2009) Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot method. Radiology 251(3):838–845
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. Am J Neuroradiol 27(4):859–867
Saini J, Gupta RK, Kumar M, Singh A, Saha I, Santosh V et al (2019) Comparative evaluation of cerebral gliomas using rCBV measurements during sequential acquisition of T1-perfusion and T2-perfusion MRI. PLoS ONE 14(4):1–14
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM et al (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusio. Am J Neuroradiol 30(3):552–558
Wetzel SG, Cha S, Johnson G, Lee P, Law M, Kasow DL et al (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224(3):797–803
Danchaivijitr N, Waldman AD, Tozer DJ, Benton CE, Caseiras GB, Tofts PS et al (2008) Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology 247(1):170–178
Mazur MD, Couldwell WT (2011) Investigating a candidate cell of origin for diffuse intrinsic pontine glioma. World Neurosurg. 76:368–373
Duchatel RJ, Jackson ER, Alvaro F, Nixon B, Hondermarck H, Dun MD (2019) Signal transduction in diffuse intrinsic pontine glioma. Proteomics. 19:e1800479
Piccardo A, Tortora D, Mascelli S, Severino M, Piatelli G, Consales A et al (2019) Advanced MR imaging and 18F-DOPA PET characteristics of H3K27M-mutant and wild-type pediatric diffuse midline gliomas. Eur J Nucl Med Mol Imaging 46(8):1685–1694
Yao R, Cheng A, Liu M, Zhang Z, Jin B, Yu H (2021) The diagnostic value of apparent diffusion coefficient and proton magnetic resonance spectroscopy in the grading of pediatric gliomas. J Comput Assist Tomogr 45(2):269–276
Lee J, Choi SH, Kim JH, Sohn CH, Lee S, Jeong J (2014) Glioma grading using apparent diffusion coefficient map: application of histogram analysis based on automatic segmentation. NMR Biomed 27(9):1046–1052
Chawla S, Kim S, Wang S, Poptani H (2009) Diffusion-weighted imaging in head and neck cancers. Futur Oncol 5(7):959–975
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Richa Singh Chauhan, Karthik Kulanthaivelu, Abhishek Kotwal, and Maya Dattatraya Bhat. The first draft of the manuscript was written by Nihar Kathrani and Richa Singh Chauhan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of the National Institute of Mental Health and Neurosciences, Bengaluru, India, approved this study.
Consent to participate
Informed consent was waived off from participants owing to its retrospective nature.
Consent for publication
Patients consent was waived off owing to the retrospective nature of the study.
Availability of data and material
Complete research data will be available whenever requested.
Code availability
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kathrani, N., Chauhan, R.S., Kotwal, A. et al. Diffusion and perfusion imaging biomarkers of H3 K27M mutation status in diffuse midline gliomas. Neuroradiology 64, 1519–1528 (2022). https://doi.org/10.1007/s00234-021-02857-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02857-x