Abstract
Purpose
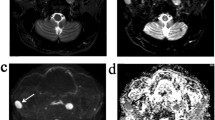
Anatomical imaging criteria for the diagnosis of malignant head and neck nodes may not always be reliable. This study aimed to evaluate the diagnostic value of conventional diffusion-weighted imaging (DWI) and intravoxel incoherent motion (IVIM) DWI in discriminating benign and malignant metastatic retropharyngeal nodes (RPNs).
Methods
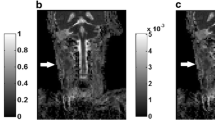
IVIM DWI using 14 b-values was performed on RPNs of 30 patients with newly diagnosed metastatic nasopharyngeal carcinoma (NPC) and 30 patients with elevated plasma Epstein-Barr virus (EBV)-DNA without NPC who were part of an EBV-based NPC screening program. Histogram measurements of the two groups were compared for pure diffusion coefficient (D), pseudo-diffusion coefficient (D*), perfusion volume fraction (f) and apparent diffusion coefficient (ADC) using the Mann-Whitney U test. Area under the curves (AUCs) of significant measurements were calculated from receiver-operating characteristics analysis and compared using the DeLong test.
Results
Compared with metastatic RPNs, benign RPNs had lower ADCmean (0.73 vs 0.82 × 10−3 mm2/s) and Dmean (0.60 vs 0.71 × 10−3 mm2/s) and a higher D*mean (35.21 vs 28.66 × 10−3 mm2/s) (all p < 0.05). There was no difference in the f measurements between the two groups (p = 0.204 to 0.301). Dmean achieved the highest AUC of 0.800, but this was not statistically better than the AUCs of the other parameters (p = 0.148 to 0.991).
Conclusion
Benign RPNs in patients with EBV-DNA showed greater restriction of diffusion compared with malignant metastatic RPNs from NPC. IVIM did not show a significant advantage over conventional DWI in discriminating benign and malignant nodes.



Similar content being viewed by others
Abbreviations
- ADC :
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- D :
-
Pure diffusion coefficient
- D*:
-
Pseudo-diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- EBV:
-
Epstein-Barr virus
- f :
-
Perfusion volume fraction
- ICC:
-
Intra-class correlation coefficient
- IQR:
-
Interquartile range
- IVIM:
-
Intravoxel incoherent motion
- MinAD:
-
Minimum axial diameter
- NPC:
-
Nasopharyngeal carcinoma
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- RPNs:
-
Retropharyngeal nodes
- SD:
-
Standard deviation
References
Coskun HH, Ferlito A, Medina JE, Robbins KT, Rodrigo JP, Strojan P, Suárez C, Takes RP, Woolgar JA, Shaha AR, de Bree R, Rinaldo A, Silver CE (2011) Retropharyngeal lymph node metastases in head and neck malignancies. Head Neck 33:1520–1529. https://doi.org/10.1002/HED
Ferlito A, Shaha AR, Rinaldo A (2002) Retropharyngeal lymph node metastasis from cancer of the head and neck. Acta Otolaryngol 122:556–560. https://doi.org/10.1080/00016480260092408
van den Brekel M, Stel H, Castelijns J et al (1990) Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 177:379–384. https://doi.org/10.1148/radiology.177.2.2217772
King AD, Ahuja AT, Leung SF, Lam WWM, Teo P, Chan YL, Metreweli C (2000) Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck 22:275–281. https://doi.org/10.1002/(SICI)1097-0347(200005)22:3<275::AID-HED10>3.0.CO;2-N
Zhang G, Liu L, Wei W et al (2010) Radiologic criteria of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma treated with radiation therapy. Radiology 255:605–612. https://doi.org/10.1148/radiol.10090289
Oikawa Y, Michi Y, Tsushima F, Tomioka H, Mochizuki Y, Kugimoto T, Osako T, Nojima H, Yokokawa M, Kashima Y, Harada H (2019) Management of retropharyngeal lymph node metastasis in oral cancer. Oral Oncol 99:104471. https://doi.org/10.1016/j.oraloncology.2019.104471
Ho FC, Tham IW, Earnest A et al (2012) Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC Cancer 12:98. https://doi.org/10.1186/1471-2407-12-98
Liu L-Z, Zhang G-Y, Xie C-M, Liu XW, Cui CY, Li L (2006) Magnetic resonance imaging of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: patterns of spread. Int J Radiat Oncol Biol Phys 66:721–730. https://doi.org/10.1016/j.ijrobp.2006.05.054
Chen YP, Chan ATC, Le QT et al (2019) Nasopharyngeal carcinoma. Lancet 394:64–80. https://doi.org/10.1016/S0140-6736(19)30956-0
Huang L, Zhang Y, Liu Y, Li H, Wang S, Liang S, Zhou J, Cui C, Sun Y, Chen M, Xu S, Li J, Liu L (2019) Prognostic value of retropharyngeal lymph node metastasis laterality in nasopharyngeal carcinoma and a proposed modification to the UICC/AJCC N staging system. Radiother Oncol 140:90–97. https://doi.org/10.1016/j.radonc.2019.04.024
Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H, Corry J, Grau C, Grégoire V, Harrington KJ, Hu CS, Kwong DL, Langendijk JA, le QT, Lee NY, Lin JC, Lu TX, Mendenhall WM, O’Sullivan B, Ozyar E, Peters LJ, Rosenthal DI, Soong YL, Tao Y, Yom SS, Wee JT (2018) International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol 126:25–36. https://doi.org/10.1016/j.radonc.2017.10.032
Lee AWM, Ma BBY, Ng WT, Chan ATC (2015) Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 33:3356–3364. https://doi.org/10.1200/JCO.2015.60.9347
Chan JYW, Chow VLY, Wong STS, Wei WI (2013) Surgical salvage for recurrent retropharyngeal lymph node metastasis in nasopharyngeal carcinoma. Head Neck 35:1726–1731. https://doi.org/10.1002/hed.23214
Ng WT, Lee MCH, Fung NTC, Wong ECY, Cheung AKW, Chow JCH, Au KH, Poon DMC, Lai JWY, Chiang CL, Choi HCW, Chau TC, Lee VHF, Lee AWM (2019) Dose volume effects of re-irradiation for locally recurrent nasopharyngeal carcinoma. Head Neck 42:1–8. https://doi.org/10.1002/hed.25988
King AD, Vlantis AC, Bhatia KSS, Zee BCY, Woo JKS, Tse GMK, Chan ATC, Ahuja AT (2011) Primary nasopharyngeal carcinoma : diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 258:531–537. https://doi.org/10.1148/radiol.10101241
King AD, Woo JKS, Ai QY, Chan JSM, Lam WKJ, Tse IOL, Bhatia KS, Zee BCY, Hui EP, Ma BBY, Chiu RWK, van Hasselt AC, Chan ATC, Lo YMD, Chan KCA (2019) Complementary roles of MRI and endoscopic examination in the early detection of nasopharyngeal carcinoma. Ann Oncol 30:977–982. https://doi.org/10.1093/annonc/mdz106
King AD, Woo JKS, Ai Q-Y, Mo FKF, So TY, Lam WKJ, Tse IOL, Vlantis AC, Yip KWN, Hui EP, Ma BBY, Chiu RWK, Chan ATC, Lo YMD, Chan KCA (2020) Early detection of cancer: evaluation of MR imaging grading systems in patients with suspected nasopharyngeal carcinoma. Am J Neuroradiol 41:515–520 https://doi.org/10.3174/ajnr.A6444
Vandecaveye V, De Keyzer F, vander Poorten V et al (2009) Head and neck squamous cell carcinoma : value of diffusion- weighted MR imaging for nodal staging. Radiology 251:134–146. https://doi.org/10.1148/radiol.2511080128
Holzapfel K, Duetsch S, Fauser C, Eiber M, Rummeny EJ, Gaa J (2009) Value of diffusion-weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur J Radiol 72:381–387. https://doi.org/10.1016/j.ejrad.2008.09.034
Jin GQ, Yang J, Liu LD et al (2016) The diagnostic value of 1.5-T diffusion-weighted MR imaging in detecting 5 to 10 mm metastatic cervical lymph nodes of nasopharyngeal carcinoma. Medicine (Baltimore) 95:e4286. https://doi.org/10.1097/MD.0000000000004286
Zhang SC, Zhou SH, Shang DS et al (2018) The diagnostic role of diffusion-weighted magnetic resonance imaging in hypopharyngeal carcinoma. Oncol Lett 15:5533–5544. https://doi.org/10.3892/ol.2018.8053
Chen C, Lin Z, Xiao Y, Bai P, Yue Q, Chen Y, Chen L (2018) Role of diffusion-weighted imaging in the discrimination of benign and metastatic parotid area lymph nodes in patients with nasopharyngeal carcinoma. Sci Rep 8:281. https://doi.org/10.1038/s41598-017-18617-y
Perrone A, Guerrisi P, Izzo L, D’Angeli I, Sassi S, Mele LL, Marini M, Mazza D, Marini M (2011) Diffusion-weighted MRI in cervical lymph nodes: differentiation between benign and malignant lesions. Eur J Radiol 77:281–286. https://doi.org/10.1016/j.ejrad.2009.07.039
Goel V, Parihar PS, Parihar A et al (2016) Accuracy of MRI in prediction of tumour thickness and nodal stage in oral tongue and gingivobuccal cancer with clinical correlation and staging. J Clin Diagn Res 10:TC01–TC05. https://doi.org/10.7860/JCDR/2016/17411.7905
Zhong J, Lu Z, Xu L, Dong L, Qiao H, Hua R, Gong Y, Liu Z, Hao C, Liu X, Zong C, He L, Liu J (2014) The diagnostic value of cervical lymph node metastasis in head and neck squamous carcinoma by using diffusion-weighted magnetic resonance imaging and computed tomography perfusion. Biomed Res Int 2014:260859–260857. https://doi.org/10.1155/2014/260859
Jović A, Fila J, Gršić K, Ivkić M, Ozretić D (2020) Diffusion-weighted MRI: impact of the size of the ROI in detecting metastases in subcentimeter lymph nodes in head and neck squamous cell carcinoma. Neuroradiology. https://doi.org/10.1007/s00234-020-02449-1
Liang L, Luo X, Lian Z, Chen W, Zhang B, Dong Y, Liang C, Zhang S (2017) Lymph node metastasis in head and neck squamous carcinoma: efficacy of intravoxel incoherent motion magnetic resonance imaging for the differential diagnosis. Eur J Radiol 90:159–165. https://doi.org/10.1016/j.ejrad.2017.02.039
Mundada P, Varoquaux AD, Lenoir V, de Vito C, Dulguerov N, Ailianou A, Caparrotti F, Becker M (2018) Utility of MRI with morphologic and diffusion weighted imaging in the detection of post-treatment nodal disease in head and neck squamous cell carcinoma. Eur J Radiol 101:162–169. https://doi.org/10.1016/j.ejrad.2018.02.026
Zhang Y, Chen J, Shen J, Zhong J, Ye R, Liang B (2013) Apparent diffusion coefficient values of necrotic and solid portion of lymph nodes: differential diagnostic value in cervical lymphadenopathy. Clin Radiol 68:224–231. https://doi.org/10.1016/j.crad.2011.04.002
Lee MC, Tsai HY, Chuang KS, Liu CK, Chen MK (2013) Prediction of nodal metastasis in head and neck cancer using a 3T MRI ADC map. Am J Neuroradiol 34:864–869. https://doi.org/10.3174/ajnr.A3281
Abdel Razek AAK, Soliman NY, Elkhamary S, Alsharaway MK, Tawfik A (2006) Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radiol 16:1468–1477. https://doi.org/10.1007/s00330-005-0133-x
Barchetti F, Pranno N, Giraldi G et al (2014) The role of 3 Tesla diffusion-weighted imaging in the differential diagnosis of benign versus malignant cervical lymph nodes in patients with head and neck squamous cell carcinoma. Biomed Res Int 2014. https://doi.org/10.1155/2014/532095
De Bondt RBJ, Hoeberigs MC, Nelemans PJ et al (2009) Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Neuroradiology 51:183–192. https://doi.org/10.1007/s00234-008-0487-2
Si J, Huang S, Shi H, Liu Z, Hu Q, Wang G, Shen G, Zhang D (2014) Usefulness of 3T diffusion-weighted MRI for discrimination of reactive and metastatic cervical lymph nodes in patients with oral squamous cell carcinoma: a pilot study. Dentomaxillofac Radiol 43:20130202. https://doi.org/10.1259/dmfr.20130202
Li H, Liu X-W, Geng Z-J, Wang DL, Xie CM (2015) Diffusion-weighted imaging to differentiate metastatic from non-metastatic retropharyngeal lymph nodes in nasopharyngeal carcinoma. Dentomaxillofac Radiol 44:20140126. https://doi.org/10.1259/dmfr.20140126
Ai QY, King AD, Chan JSM, Chen W, Chan KCA, Woo JKS, Zee BCY, Chan ATC, Poon DMC, Ma BBY, Hui EP, Ahuja AT, Vlantis AC, Yuan J (2019) Distinguishing early-stage nasopharyngeal carcinoma from benign hyperplasia using intravoxel incoherent motion diffusion-weighted MRI. Eur Radiol 29:5627–5634. https://doi.org/10.1007/s00330-019-06133-8
Zhang SX, Jia QJ, Zhang ZP, Liang CH, Chen WB, Qiu QH, Li H (2014) Intravoxel incoherent motion MRI: emerging applications for nasopharyngeal carcinoma at the primary site. Eur Radiol 24:1998–2004. https://doi.org/10.1007/s00330-014-3203-0
Noij DP, Martens RM, Marcus JT, de Bree R, Leemans CR, Castelijns JA, de Jong MC, de Graaf P (2017) Intravoxel incoherent motion magnetic resonance imaging in head and neck cancer: a systematic review of the diagnostic and prognostic value. Oral Oncol 68:81–91. https://doi.org/10.1016/j.oraloncology.2017.03.016
Hejduk B, Bobek-Billewicz B, Rutkowski T et al (2017) Application of intravoxel incoherent motion (IVIM) model for differentiation between metastatic and non-metastatic head and neck lymph nodes. Pol J Radiol 82:506–510. https://doi.org/10.12659/PJR.902275
Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, Chu SWI, Mak C, Tse IOL, Leung SYM, Chan G, Hui EP, Ma BBY, Chiu RWK, Leung SF, van Hasselt AC, Chan ATC, Lo YMD (2017) Analysis of plasma Epstein–Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 377:513–522. https://doi.org/10.1056/NEJMoa1701717
Thoeny HC, De Keyzer F, King AD (2012) Diffusion-weighted MR imaging in the head and neck. Radiology 263:19–32. https://doi.org/10.1148/radiol.11101821
Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228:303–308. https://doi.org/10.1148/radiol.2282011860
Kerr JR (2019) Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol clinpath-2019-205822. https://doi.org/10.1136/jclinpath-2019-205822
Sumi M, Sakihama N, Sumi T, Morikawa M, Uetani M, Kabasawa H, Shigeno K, Hayashi K, Takahashi H, Nakamura T (2003) Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. Am J Neuroradiol 24:1627–1634
Sumi M, Van Cauteren M, Nakamura T (2006) MR microimaging of benign and malignant nodes in the neck. Am J Roentgenol 186:749–757. https://doi.org/10.2214/AJR.04.1832
Wendl CM, Müller S, Eiglsperger J, Fellner C, Jung EM, Meier JK (2016) Diffusion-weighted imaging in oral squamous cell carcinoma using 3 Tesla MRI: is there a chance for preoperative discrimination between benign and malignant lymph nodes in daily clinical routine? Acta Radiol 57:939–946. https://doi.org/10.1177/0284185115609365
Cintra MB, Ricz H, Mafee MF, dos Santos AC (2018) Contribution of dynamic contrast enhancement and diffusion-weighted magnetic resonance imaging to the diagnosis of malignant cervical lymph nodes. Radiol Bras 51:IX. https://doi.org/10.1590/0100-3984.2018.51.3e3
King AD, Ahuja AT, Yeung DKW, Fong DKY, Lee YYP, Lei KIK, Tse GMK (2007) Malignant cervical lymphadenopathy : diagnostic accuracy of diffusion-weighted MR imaging. Radiology 245:806–813. https://doi.org/10.1148/radiol.2451061804
Vidiri A, Marzi S, Gangemi E, Benevolo M, Rollo F, Farneti A, Marucci L, Spasiano F, Sperati F, di Giuliano F, Pellini R, Sanguineti G (2019) Intravoxel incoherent motion diffusion-weighted imaging for oropharyngeal squamous cell carcinoma: correlation with human papillomavirus status. Eur J Radiol 119:108640. https://doi.org/10.1016/j.ejrad.2019.08.009
Lee FK, King AD, Ma BB-Y, Yeung DK (2012) Dynamic contrast enhancement magnetic resonance imaging (DCE-MRI) for differential diagnosis in head and neck cancers. Eur J Radiol 81:784–788
Razek AAKA, Elsorogy LG, Soliman NY, Nada N (2011) Dynamic susceptibility contrast perfusion MR imaging in distinguishing malignant from benign head and neck tumors: a pilot study. Eur J Radiol 77:73–79. https://doi.org/10.1016/j.ejrad.2009.07.022
Kang KM, Choi SH, Kim DE, Yun TJ, Kim JH, Sohn CH, Park SW (2017) Application of cardiac gating to improve the reproducibility of intravoxel incoherent motion measurements in the head and neck. Magn Reson Med Sci 16:190–202. https://doi.org/10.2463/mrms.mp.2016-0051
Xiao B, Wang P, Zhao Y, Liu Y, Ye Z (2020) Nasopharyngeal carcinoma perfusion MRI: comparison of arterial spin labeling and dynamic contrast-enhanced MRI. Medicine (Baltimore) 99:e20503. https://doi.org/10.1097/MD.0000000000020503
Lin M, Yu X, Luo D, Ouyang H, Xie L, Wu B, Zhou C (2018) Investigating the correlation of arterial spin labeling and dynamic contrast enhanced perfusion in primary tumor of nasopharyngeal carcinoma. Eur J Radiol 108:222–229. https://doi.org/10.1016/j.ejrad.2018.09.034
Yun TJ, Sohn CH, Han MH, Kang HS, Kim JE, Yoon BW, Paeng JC, Choi SH, Kim JH, Song IC, Chang KH (2013) Effect of delayed transit time on arterial spin labeling: correlation with dynamic susceptibility contrast perfusion magnetic resonance in moyamoya disease. Investig Radiol 48:795–802. https://doi.org/10.1097/RLI.0b013e3182981137
Sun Q, Ma C, Dong M et al (2019) Effects of region of interest sizes on apparent diffusion coefficient measurements of pleomorphic adenoma, Warthin tumor, and normal parotid parenchyma. Quant Imaging Med Surg 9:681–690. https://doi.org/10.21037/qims.2019.04.11
Funding
The work described in this paper was supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No.: 14107216).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
This is a retrospective study with a waiver of informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
So, T.Y., Ai, QY.H., Lam, W.K.J. et al. Intravoxel incoherent motion diffusion-weighted imaging for discrimination of benign and malignant retropharyngeal nodes. Neuroradiology 62, 1667–1676 (2020). https://doi.org/10.1007/s00234-020-02494-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02494-w