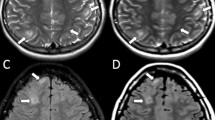
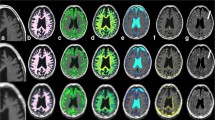
Abstract
Purpose
Accelerated myelination in the affected hemisphere has been demonstrated previously in patients with Sturge-Weber syndrome (SWS). This prospective study investigated myelin-related changes in patients with unilateral SWS using synthetic quantitative magnetic resonance imaging (qMRI).
Methods
Fourteen children with unilateral SWS were categorized according to age, i.e., ≤ 2 years (group A, n = 5, mean age 1.1 years, 3 males) and > 2 years (group B, n = 9, mean age 3.9 years, 4 males). All children underwent two-dimensional synthetic qMRI. The myelin volume in the cerebral hemisphere and white matter (WM) myelin volume fraction (MVF), proton density (PD), R1 and R2 relaxation rates ipsilateral to the leptomeningeal enhancement, and/or a port-wine birthmark were compared with the corresponding values in the contralateral hemisphere.
Results
In group A, 3 patients had a higher myelin volume in the ipsilateral hemisphere and a higher MVF, R1, and R2 and lower PD in the ipsilateral WM than on the contralateral side; the findings were the opposite in the remaining two patients. All patients in group B had a significantly lower myelin volume in the ipsilateral hemisphere (P < 0.05) and a lower MVF and R1 and higher PD in the ipsilateral WM than on the contralateral side (P < 0.0125).
Conclusion
Higher estimated myelin was observed on the ipsilateral side in some patients aged ≤ 2 years and lower myelin on the ipsilateral side in all older patients. Synthetic qMRI might be useful for showing myelin-related abnormalities in SWS.






Similar content being viewed by others
References
Lo W, Marchuk DA, Ball KL, Juhasz C, Jordan LC, Ewen JB, Comi A, Brain Vascular Malformation Consortium National Sturge-Weber Syndrome W (2012) Updates and future horizons on the understanding, diagnosis, and treatment of Sturge-Weber syndrome brain involvement. Dev Med Child Neurol 54(3):214–223. https://doi.org/10.1111/j.1469-8749.2011.04169.x
Sundaram SK, Michelhaugh SK, Klinger NV, Kupsky WJ, Sood S, Chugani HT, Mittal S, Juhasz C (2017) GNAQ mutation in the venous vascular malformation and underlying brain tissue in Sturge-Weber syndrome. Neuropediatrics 48(5):385–389. https://doi.org/10.1055/s-0037-1603515
Alkonyi B, Govindan RM, Chugani HT, Behen ME, Jeong JW, Juhasz C (2011) Focal white matter abnormalities related to neurocognitive dysfunction: an objective diffusion tensor imaging study of children with Sturge-Weber syndrome. Pediatr Res 69(1):74–79. https://doi.org/10.1203/PDR.0b013e3181fcb285
Adamsbaum C, Pinton F, Rolland Y, Chiron C, Dulac O, Kalifa G (1996) Accelerated myelination in early Sturge-Weber syndrome: MRI-SPECT correlations. Pediatr Radiol 26(11):759–762
Andica C, Hagiwara A, Nakazawa M, Tsuruta K, Takano N, Hori M, Suzuki H, Sugano H, Arai H, Aoki S (2016) The advantage of synthetic MRI for the visualization of early white matter change in an infant with Sturge-Weber syndrome. Magn Reson Med Sci 15(4):347–348. https://doi.org/10.2463/mrms.ci.2015-0164
George U, Rathore S, Nittala P (2010) MR demonstration of accelerated myelination in early Sturge Weber syndrome. Neurol India 58(2):336–337. https://doi.org/10.4103/0028-3886.63769
Hagiwara A, Andica C, Hori M, Aoki S (2017) Synthetic MRI showed increased myelin partial volume in the white matter of a patient with Sturge-Weber syndrome. Neuroradiology 59(11):1065–1066. https://doi.org/10.1007/s00234-017-1908-x
Jacoby CG, Yuh WT, Afifi AK, Bell WE, Schelper RL, Sato Y (1987) Accelerated myelination in early Sturge-Weber syndrome demonstrated by MR imaging. J Comput Assist Tomogr 11(2):226–231
Moritani T, Kim J, Sato Y, Bonthius D, Smoker WR (2008) Abnormal hypermyelination in a neonate with Sturge-Weber syndrome demonstrated on diffusion-tensor imaging. J Magn Reson Imaging 27(3):617–620. https://doi.org/10.1002/jmri.21248
Pfund Z, Kagawa K, Juhasz C, Shen C, Lee JS, Chugani DC, Muzik O, Chugani HT (2003) Quantitative analysis of gray- and white-matter volumes and glucose metabolism in Sturge-Weber syndrome. J Child Neurol 18(2):119–126. https://doi.org/10.1177/08830738030180021501
Porto L, Kieslich M, Yan B, Zanella FE, Lanfermann H (2006) Accelerated myelination associated with venous congestion. Eur Radiol 16(4):922–926. https://doi.org/10.1007/s00330-005-0044-x
Adams ME, Aylett SE, Squier W, Chong W (2009) A spectrum of unusual neuroimaging findings in patients with suspected Sturge-Weber syndrome. AJNR Am J Neuroradiol 30(2):276–281. https://doi.org/10.3174/ajnr.A1350
Comi AM, Sahin M, Hammill A, Kaplan EH, Juhasz C, North P, Ball KL, Levin AV, Cohen B, Morris J, Lo W, Roach ES, Sturge-Weber Syndrome Research W (2016) Leveraging a Sturge-Weber gene discovery: an agenda for future research. Pediatr Neurol 58:12–24. https://doi.org/10.1016/j.pediatrneurol.2015.11.009
Sled JG, Nossin-Manor R (2013) Quantitative MRI for studying neonatal brain development. Neuroradiology 55(Suppl 2):97–104. https://doi.org/10.1007/s00234-013-1235-9
Hagiwara A, Warntjes M, Hori M, Andica C, Nakazawa M, Kumamaru KK, Abe O, Aoki S (2017) SyMRI of the brain: rapid quantification of relaxation rates and proton density, with synthetic MRI, automatic brain segmentation, and myelin measurement. Investig Radiol 52(10):647–657. https://doi.org/10.1097/RLI.0000000000000365
Hagiwara A, Hori M, Cohen-Adad J, Nakazawa M, Suzuki Y, Kasahara A, Horita M, Haruyama T, Andica C, Maekawa T, Kamagata K, Kumamaru KK, Abe O, Aoki S (2019) Linearity, bias, intrascanner repeatability, and interscanner reproducibility of quantitative multidynamic multiecho sequence for rapid simultaneous relaxometry at 3 T: a validation study with a standardized phantom and healthy controls. Investig Radiol 54:39–47. https://doi.org/10.1097/RLI.0000000000000510
Andica C, Hagiwara A, Hori M, Nakazawa M, Goto M, Koshino S, Kamagata K, Kumamaru KK, Aoki S (2018) Automated brain tissue and myelin volumetry based on quantitative MR imaging with various in-plane resolutions. J Neuroradiol 45(3):164–168. https://doi.org/10.1016/j.neurad.2017.10.002
Warntjes JBM, Persson A, Berge J, Zech W (2017) Myelin detection using rapid quantitative MR imaging correlated to macroscopically registered Luxol fast blue-stained brain specimens. AJNR Am J Neuroradiol 38(6):1096–1102. https://doi.org/10.3174/ajnr.A5168
Hagiwara A, Hori M, Kamagata K, Warntjes M, Matsuyoshi D, Nakazawa M, Ueda R, Andica C, Koshino S, Maekawa T, Irie R, Takamura T, Kumamaru KK, Abe O, Aoki S (2018) Myelin measurement: comparison between simultaneous tissue relaxometry, magnetization transfer saturation index, and T1w/T2w ratio methods. Sci Rep 8(1):10554. https://doi.org/10.1038/s41598-018-28852-6
Hagiwara A, Hori M, Yokoyama K, Nakazawa M, Ueda R, Horita M, Andica C, Abe O, Aoki S (2017) Analysis of white matter damage in patients with multiple sclerosis via a novel in vivo magnetic resonance method for measuring myelin, axons, and G-ratio. AJNR Am J Neuroradiol 38:1934–1940
Wallaert L, Hagiwara A, Andica C, Hori M, Yamashiro K, Koshino S, Maekawa T, Kamagata K, Aoki S (2017) The advantage of synthetic MRI for the visualization of anterior temporal pole lesions on double inversion recovery (DIR), phase-sensitive inversion recovery (PSIR), and myelin images in a patient with CADASIL. Magn Reson Med Sci 17:275–276. https://doi.org/10.2463/mrms.ci.2017-0110
Tanenbaum LN, Tsiouris AJ, Johnson AN, Naidich TP, DeLano MC, Melhem ER, Quarterman P, Parameswaran SX, Shankaranarayanan A, Goyen M, Field AS (2017) Synthetic MRI for clinical neuroimaging: results of the MAGnetic resonance image Compilation (MAGiC) prospective, multicenter, multireader trial. AJNR Am J Neuroradiol 38(6):1103–1110. https://doi.org/10.3174/ajnr.A5227
West H, Leach JL, Jones BV, Care M, Radhakrishnan R, Merrow AC, Alvarado E, Serai SD (2017) Clinical validation of synthetic brain MRI in children: initial experience. Neuroradiology 59(1):43–50. https://doi.org/10.1007/s00234-016-1765-z
Kim HG, Moon WJ, Han J, Choi JW (2017) Quantification of myelin in children using multiparametric quantitative MRI: a pilot study. Neuroradiology 59:1043–1051. https://doi.org/10.1007/s00234-017-1889-9
Lee SM, Choi YH, You SK, Lee WK, Kim WH, Kim HJ, Lee SY, Cheon H (2018) Age-related changes in tissue value properties in children: simultaneous quantification of relaxation times and proton density using synthetic magnetic resonance imaging. Investig Radiol 53(4):236–245. https://doi.org/10.1097/RLI.0000000000000435
McAllister A, Leach J, West H, Jones B, Zhang B, Serai S (2017) Quantitative synthetic MRI in children: normative intracranial tissue segmentation values during development. AJNR Am J Neuroradiol 38(12):2364–2372. https://doi.org/10.3174/ajnr.A5398
Serai S, Dudley J, Leach J (2019) Comparison of whole brain segmentation and volume estimation in children and young adults using SPM and SyMRI. Clin Imaging 57:77–82. https://doi.org/10.1016/j.clinimag.2019.05.008
Andica C, Hagiwara A, Hori M, Kamagata K, Koshino S, Maekawa T, Suzuki M, Fujiwara H, Ikeno M, Shimizu T, Suzuki H, Sugano H, Arai H, Aoki S (2019) Review of synthetic MRI in pediatric brains: basic principle of MR quantification, its features, clinical applications, and limitations. J Neuroradiol 46:268–275. https://doi.org/10.1016/j.neurad.2019.02.005
Barkovich AJ (2000) Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol 21(6):1099–1109
Alkonyi B, Chugani HT, Juhasz C (2011) Transient focal cortical increase of interictal glucose metabolism in Sturge-Weber syndrome: implications for epileptogenesis. Epilepsia 52(7):1265–1272. https://doi.org/10.1111/j.1528-1167.2011.03066.x
Cheepsunthorn P, Palmer C, Menzies S, Roberts RL, Connor JR (2001) Hypoxic/ischemic insult alters ferritin expression and myelination in neonatal rat brains. J Comp Neurol 431(4):382–396
Hamilton SP, Rome LH (1994) Stimulation of in vitro myelin synthesis by microglia. Glia 11(4):326–335. https://doi.org/10.1002/glia.440110405
Rischke R, Krieglstein J (1991) Postischemic neuronal damage causes astroglial activation and increase in local cerebral glucose utilization of rat hippocampus. J Cereb Blood Flow Metab 11(1):106–113. https://doi.org/10.1038/jcbfm.1991.12
John F, Maqbool M, Jeong JW, Agarwal R, Behen ME, Juhasz C (2018) Deep cerebral vein expansion with metabolic and neurocognitive recovery in Sturge-Weber syndrome. Ann Clin Transl Neurol 5(4):502–506. https://doi.org/10.1002/acn3.546
Roach ES (1992) Neurocutaneous syndromes. Pediatr Clin N Am 39(4):591–620
Waelchli R, Aylett SE, Robinson K, Chong WK, Martinez AE, Kinsler VA (2014) New vascular classification of port-wine stains: improving prediction of Sturge-Weber risk. Br J Dermatol 171(4):861–867. https://doi.org/10.1111/bjd.13203
Plateroti AM, Plateroti R, Mollo R, Librando A, Contestabile MT, Fenicia V (2017) Sturge-Weber syndrome associated with monolateral ocular melanocytosis, iris mammillations, and diffuse choroidal haemangioma. Case Rep Ophthalmol 8(2):375–384. https://doi.org/10.1159/000477612
Yamaguchi K, Lonic D, Chen C, Lo LJ (2016) Correction of facial deformity in Sturge-Weber syndrome. Plast Reconstr Surg Glob Open 4(8):e843. https://doi.org/10.1097/GOX.0000000000000843
Warne RR, Carney OM, Wang G, Bhattacharya D, Chong WK, Aylett SE, Mankad K (2018) The bone does not predict the brain in Sturge-Weber syndrome. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5722
Sujansky E, Conradi S (1995) Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol 10(1):49–58. https://doi.org/10.1177/088307389501000113
Lee JS, Asano E, Muzik O, Chugani DC, Juhasz C, Pfund Z, Philip S, Behen M, Chugani HT (2001) Sturge-Weber syndrome: correlation between clinical course and FDG PET findings. Neurology 57(2):189–195
Jagtap SA, Srinivas G, Radhakrishnan A, Harsha KJ (2013) A clinician’s dilemma: Sturge-Weber syndrome ‘without facial nevus’!! Ann Indian Acad Neurol 16(1):118–120. https://doi.org/10.4103/0972-2327.107725
Arulrajah S, Ertan G, A MC, Tekes A, Lin DL, Huisman TA (2010) MRI with diffusion-weighted imaging in children and young adults with simultaneous supra- and infratentorial manifestations of Sturge-Weber syndrome. J Neuroradiol 37(1):51–59. https://doi.org/10.1016/j.neurad.2009.05.001
Guseo A (1975) Ultrastructure of calcification in Sturge-Weber disease. Virchows Arch A Pathol Anat Histol 366(4):353–356
Wu J, Tarabishy B, Hu J, Miao Y, Cai Z, Xuan Y, Behen M, Li M, Ye Y, Shoskey R, Haacke EM, Juhasz C (2011) Cortical calcification in Sturge-Weber syndrome on MRI-SWI: relation to brain perfusion status and seizure severity. J Magn Reson Imaging 34(4):791–798. https://doi.org/10.1002/jmri.22687
Alkonyi B, Chugani HT, Behen M, Halverson S, Helder E, Makki MI, Juhasz C (2010) The role of the thalamus in neuro-cognitive dysfunction in early unilateral hemispheric injury: a multimodality imaging study of children with Sturge-Weber syndrome. Eur J Paediatr Neurol 14(5):425–433. https://doi.org/10.1016/j.ejpn.2010.03.012
Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG (2011) Mapping infant brain myelination with magnetic resonance imaging. J Neurosci 31(2):784–791. https://doi.org/10.1523/JNEUROSCI.2106-10.2011
Acknowledgments
We thank all parents and patients who participated in this study.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant numbers 16K19852 and 16K10327); by a JSPS Grant-in-Aid for Scientific Research on Innovative Areas, resource and technical support platforms for promoting research “Advanced Bioimaging Support” (Grant number JP16H06280); by the Japanese Society for Magnetic Resonance in Medicine; by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and Development (AMED); by the AMED under Grant number JP18lk1010025; and by the Impulsing Paradigm Change through Disruptive Technologies (ImPACT) Program of the Council for Science, Technology and Innovation (Cabinet Office, Government of Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in reports involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants before evaluation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andica, C., Hagiwara, A., Hori, M. et al. Aberrant myelination in patients with Sturge-Weber syndrome analyzed using synthetic quantitative magnetic resonance imaging. Neuroradiology 61, 1055–1066 (2019). https://doi.org/10.1007/s00234-019-02250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02250-9