Abstract
Introduction
Limbic encephalitis (LE) associated with voltage-gated potassium channel-complex antibodies (VGKC-LE) is frequently non-paraneoplastic and associated with marked improvement following corticosteroid therapy. Mesial temporal lobe abnormalities are present in around 80 % of patients. If associated or preceded by faciobrachial dystonic seizures, basal ganglia signal changes may occur. In some patients, blurring of the supratentorial white matter on T2-weighted images (SWMB) may be seen. The purpose of this study was to evaluate the incidence of SWMB and whether it is specific for VGKC-LE.
Methods
Two experienced neuroradiologists independently evaluated signal abnormalities on FLAIR MRI in 79 patients with LE while unaware on the antibody type.
Results
SWMB was independently assessed as present in 10 of 36 (28 %) compared to 2 (5 %) of 43 non-VGKC patients (p = 0.009). It was not related to the presence of LGI1 or CASPR2 proteins of VGKC antibodies. MRI showed increased temporomesial FLAIR signal in 22 (61 %) VGKC compared to 14 (33 %) non-VGKC patients (p = 0.013), and extratemporomesial structures were affected in one VGKC (3 %) compared to 11 (26 %) non-VGKC patients (p = 0.005).
Conclusion
SWMB is a newly described MRI sign rather specific for VGKC-LE.
Similar content being viewed by others
Limbic encephalitis (LE) is a clinicopathological entity initially described in the 1960s [1,2]. Patients present with subacute (days to weeks) memory deficits, epileptic seizures, and/or mood and behavioral changes; brain specimens show inflammatory changes most pronounced in limbic structures, e.g., hippocampus and amygdala. While initially related to cancer and onconeuronal antibodies (e.g., Hu, Yo, Ri, Ma2, Tr, CV2, amphiphysin), in recent years, LE with antibodies against different cell surface proteins were recognized. These antibodies are targeted against ion channels, receptors, or proteins associated with the voltage-gated potassium channel-complex (VGKC), i.e., leucine-rich glioma inactivated 1 (LGI1) or contactin-associated protein-like 2 (CASPR2) protein, N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), or γ-aminobutyric acid B (GABAB) receptors. In contrast to paraneoplastic LE, they occur with and without underlying tumor and frequently respond well to immunotherapy [3,4].
MRI features of non-paraneoplastic LE include increased T2 signal of temporomesial structures, namely amygdala and hippocampus. Initially, these structures are swollen; within weeks to months, swelling may disappear and progressive atrophy may occur [5,6]. The predominant involvement of the amygdalae is considered typical for LE [6,7]. Other gray matter structures (e.g., basal ganglia, cerebellum, thalamus, cerebral cortex) may be affected in non-paraneoplastic LE; a specific MRI feature has not been described so far. After identifying an increased T2 signal of the supratentorial white matter (supratentorial white matter blurring (SWMB)) in several limbic encephalitis patients with antibodies to the VGKC complex (henceforth, VGKC encephalitis), we sought to evaluate whether this is a specific feature and whether it differs in the subtypes with LGI1 and CASPR2 antibodies.
Methods
MRI scans of 79 patients with LE were collected from six university hospitals in Germany and Austria by a neurologist (A.B.). LE was diagnosed based on the features of (1) limbic signs and symptoms in adolescence or adulthood (≥one of the following: seizures of temporal semiology, disturbance of episodic memory, psychiatric symptoms with affective, and/or anxiety disturbances) and (2) presence of serum antibodies associated with LE (i.e., onconeural, VGKC, GAD, NMDA, GABAB, AMPA) [6]. VGKC antibodies were assessed by radioimmunoprecipitation assay (RIA; normal values <100 pmol/l) or by indirect immunofluorescence using formalin-fixed HEK293 cells containing LGI1 and CASPR2 proteins [8]. Some university hospitals used both methods.
Patients had been studied between 2005 and 2014 on nine different 1.5- and 3-T scanners with different MRI protocols which included at least axial or coronal FLAIR sequences with 5-mm-thick slices. All data were pseudonymously stored on a PACS system and evaluated by two board-certified neuroradiologists (I.M., H.U.) who knew that these patients had antibody-proven LE but were not aware of the antibody type.
Both neuroradiologists independently assessed the following MRI criteria and rated those as present or absent:
-
1)
increased FLAIR signal of temporomesial (amygdala and/or hippocampus) cortex
-
2)
increased FLAIR signal of extratemporomesial cortices
-
3)
homogenously increased FLAIR signal of the supratentorial white matter rendering the differentiation between white and cortex more difficult
MRI criteria were considered present for the final analysis if both raters agreed. If there was disagreement or unequivocal absence, the criteria were considered absent.
An institutional review board approval was obtained for this retrospective study, and informed consent was waived.
Statistics
In order to evaluate whether cortical FLAIR signal and supratentorial white matter blurring differed in VGKC and non-VGKC patients, Fisher’s exact tests were calculated.
To evaluate the inter-observer agreement for both, cortical FLAIR signal and supratentorial white matter blurring, and Kappa coefficients were calculated.
Results
Group 1 (VGKC encephalitis) comprised 36 patients (22 males, 19 to 86 (mean 58) years). In 16 patients, antibodies were of the LGI1 subtype, in six patients of the CASPR2 subtype, and in 14 patients, no subtype could be specified, respectively.
Group 2 (non-VGKC encephalitis) comprised 43 patients (12 males, 7 to 79 (mean 41) years) with the following antibodies: GABAB, n = 1; GAD, n = 9; Hu, n = 4; Ri, n = 2; Yo, n = 1; MA2, n = 1; mGluR5, n = 1; and NMDA, n = 24.
FLAIR signal of the temporomesial cortex was increased in 22 (61 %) VGKC compared to 14 (33 %) non-VGKC patients (p = 0.013) (Fig. 1a); extratemporomesial cortices were affected in one VGKC (3 %) compared to 11 (26 %) non-VGKC patients, respectively (p = 0.005) (Fig. 1b). Both readers agreed in 64 and differed in 15 of 79 patients, respectively (Kappa 0.708).
Supratentorial white matter blurring (SWMB) was independently assessed as present in 10 of 36 (28 %) compared to 2 (5 %) of 43 non-VGKC patients (p = 0.009) (Figs. 1c, 2, and 3). It was present in 4 of 16 patients with LGI1 and in two of six patients with CASPR2 proteins of VGKC antibodies, respectively. Both observers agreed in 58 and differed in 21 of 79 patients, respectively (Kappa 0.596).
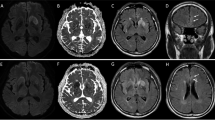
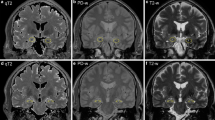
a–c A 67-year-old man with VGKC encephalitis (CASPR2 antibodies) shows increased FLAIR signal of the amygdalae (a arrows) and reduced gray white matter contrast of both hemispheres (supratentorial white matter blurring SWMB) (b, c arrows). d–f A 71-year-old woman with VGKC encephalitis shows markedly enlarged and swollen amygdalae (d arrows) and SWMB (e, f arrows). g–i In a 19-year-old woman with VGKC encephalitis amygdalae are enlarged and hyperintense (not shown). Due to the distinct SWMB (h arrows) T2-hypointensity of the globus pallidus and substantia nigra gets more evident (g, i arrow)
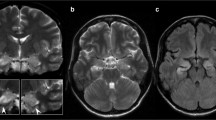
a–c A 26-year-old woman with NMDA encephalitis shows increased FLAIR signal intensity of the right pulvinar thalami (a, c arrow), but no white matter blurring. d, e A 19-year-old woman with encephalitis due to glutamic acid decarboxylase (GAD) antibodies shows increased FLAIR signal intensity of the right hippocampus (d, f arrow) and of cortex in the depth of parietal sulci (e arrows), but no white matter blurring. g–i A 61-year-old woman with NMDA encephalitis shows increased FLAIR signal intensity of the left hippocampus (i arrow) and discrete white matter blurring of the supratentorial white matter (g, h arrow)
In a post hoc analysis, we compared VGKC patients with non-VGKC patients harboring only cell surface antibodies (n = 26). In the non-VGKC group with cell surface antibodies, FLAIR signal of temporomesial cortex was increased in 11/26 (42 %) patients (n.s.); FLAIR signal of extratemporomesial cortices was increased in 3/26 (12 %) patients (n.s.), and SWMB was absent (p = 0.017).
Discussion
SWMB is a not sensitive but rather specific sign of limbic encephalitis with VGKC complex encephalitis (VGKC encephalitis). Recognizing this sign may guide the clinician to a faster therapy since patients with VGKC encephalitis may better respond to corticosteroid therapy than patients with other non-paraneoplastic limbic encephalitis [9–12].
SWMB is not related to the antibody subtype (LGI1 or CASPR2 proteins of the VGKC complex), although both subtypes differ clinically and on MRI.
Patients with the LGI1 subtype typically present with subacute memory disturbances and neuropsychiatric symptoms, such as disorientation, confusion, temporal lobe seizures, and behavioral abnormalities. Men (typically older than 40 years) are more frequently affected than women [8,13]. LGI1 encephalitis is often preceded by faciobrachial dystonic seizures (FBDS); early immunotherapy may prevent progression to LE [14,15]. MRI at the FBDS stage is unremarkable or it shows basal ganglia lesions. Hyperintense T2 signal changes, restricted diffusion and/or contrast-enhancing lesions of the caudate head and globus pallidus contralateral to the FBDS have been described [15–18]. At the LE stage, around 80 % of patients with LGI1 encephalitis show increased T2 signal intensity of one or both medial temporal lobes; basal ganglia signal changes have been described [8,13–15]. Patients frequently develop hippocampal sclerosis and remain with permanent general cognitive and mnestic deficits [5,11,19–22].
Patients with the CASPR2 subtype (almost exclusively men) develop Morvan’s syndrome (encephalopathy with prominent psychiatric symptoms, insomnia, dysautonomia, and neuromyotonia) or, less frequently, LE [8,23]. MRI is typically normal, if patients only have Morvan’s syndrome. If they have LE, around one third of patients show hyperintense T2 signal in the medial temporal lobes [8]. In contrast to LGI1-positive patients, patients with CASPR2 antibodies do not develop hippocampal sclerosis [11,21].
Due to the large variability of applied MRI protocols, a detailed assessment of MRI changes in this retrospective study was not possible. However, 61 % of patients with VGKC encephalitis showed T2-hyperintense signal changes of the medial temporal lobes which are concordant with previous reports (Table 1). Basal ganglia changes, restricted diffusion, and/or contrast enhancement were not observed, although only some patients had received contrast material.
That mesiotemporal structures show MRI changes in VGKC encephalitis is explained with the fact that hippocampus and associated limbic structures express high levels of potassium channels [24]. Our study shows that temporomesial involvement is common in VGKC and non-VGKC patients with cell surface antibodies, but rarer in patients with intracellular antibodies. In contrast, extratemporomesial involvement is more common in patients with intracellular antibodies.
SWMB is significantly more common in VGKC patients than in non-VGKC patients (p = 0.009), and this difference persists if patients with intracellular antibodies only are compared (p = 0.017). Why SWMB has not been described so far is unclear. It may be related to the fact that many patients in this and former studies were evaluated with fast MRI protocols on 1.5-T scanners (e.g., stroke protocols including axial DWI, axial and/or coronal FLAIR, axial T2* or SWI, axial 3D-TOF sequences) but not with epilepsy-dedicated MRI protocols, in which high-resolution FLAIR sequences have the highest diagnostic sensitivity [25,26]. However, SWMB can be seen on 1.5-T scanners as shown in Fig. 2a on a 3-mm coronal FLAIR and an axial 5-mm T2-weighted fast spin echo sequence. In order to avoid over-interpretation of the subtle MRI finding SWMB and acknowledging the fact that both readers in this retrospective multicenter study knew patients had antibody-proven LE, we chose only to count SWMB as present if two readers independently assessed the signal of the supratentorial white matter as increased. No consensus reading was attempted, and a questionably increased T2 signal was not counted.
Why VGKC encephalitis patients have SWMB is less clear: MRI findings are compatible with hypo- rather than with demyelination, although VGKC complex antibodies have been found in children with demyelinating syndrome (acute disseminated encephalomyelitis (ADEM), optic neuritis, clinically isolated syndrome (CIS)) [27]. Knock-out of the LGI1 gene results in hypomyelination in mice [28]. CASPR2 is expressed in the juxtaparanodal regions of myelinated fibers in the CNS and PNS [29]. Thus, anti-LGI1 as well as anti-CAPSR2 autoimmunity might lead to structural myelin changes and thereby to the observed signal abnormalities detected by MRI.
Conclusion
Supratentorial white matter blurring (SWMB) occurs in 28 % of patients with VGKC complex encephalitis and is rather specific for this in most cases non-paraneoplastic limbic encephalitis type.
References
Brierley JB, Corsellis JAN, Hierons R, Nevin S (1960) Subacute encephalitis of later adult life. Mainly affecting the limbic areas. Brain 83:357–368
Corsellis JAN, Goldberg GJ, Norton AR (1968) Limbic encephalitis and its association with carcinoma. Brain 91:481–496
Dalmau J, Rosenfeld MR (2014) Autoimmune encephalitis update. Neuro Oncol 16:771–778
Irani SR, Gelfand JM, Bettcher BM, Singhal NS, Geschwind MD (2014) Effect of rituximab in patients with leucine-rich, glioma-inactivated 1 antibody- associated encephalopathy. JAMA Neurol 71:896–900
Urbach H, Soeder BM, Jeub M, Klockgether T, Meyer B, Bien CG (2006) Serial MRI of limbic encephalitis. Neuroradiology 48:380–386
Wagner J, Witt JA, Helmstaedter C, Malter MP, Weber B, Elger CE (2015) Automated volumetry of the mesiotemporal structures in antibody-associated limbic encephalitis. J Neurol Neurosurg Psychiatry 86:735–742
Wagner J, Schoene-Bake J-C, Malter MP, Urbach H, Huppertz HJ, Elger CE, Weber B (2013) Quantitative FLAIR analysis indicates predominant affection of the amygdala in antibody-associated limbic encephalitis. Epilepsia 54:1679–1687
Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A (2010) Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 133:2734–2748
Soeder BM, Urbach H, Elger CE, Bien CG, Beyenburg S (2005) VGKC antibodies associated with limbic encephalitis. Nervenarzt 76:760–762
Soeder BM, Gleissner U, Urbach H, Clusmann H, Elger CE, Vincent A, Bien CG (2009) Causes, presentation and outcome of lesional adult onset mediotemporal lobe epilepsy. J Neurol Neurosurg Psychiatry 80:894–899
Malter MP, Frisch C, Schoene-Bake JC, Helmstaedter C, Wandinger KP, Stoecker W, Urbach H, Surges R, Elger CE, Vincent AV, Bien CG (2014) Outcome of limbic encephalitis with VGKC-complex antibodies: relation to antigenic specificity. J Neurol 261:1695–1705
Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG (2010) Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol 67:470–478
Lai M, Huijbers MGM, Lancaster E, Graus F, Bataller L, Balice-Gordon R, Cowell JK, Dalmau J (2010) Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 9:776–785
Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, Schott JM, Armstrong RJ, Zagami S, Bleasel A, Somerville ER, Smith SM, Vincent A (2011) Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 69:892–900
Irani SR, Stagg CJ, Schott JM, Rosenthal CR, Schneider SA, Pettingill P, Pettingill R, Waters P, Thomas A, Voets NL, Cardoso MJ, Cash DM, Manning EN, Lang B, Smith SJM, Vincent A, Johnson MR (2013) Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 136:3151–3162
Plantone D, Renna R, Grossi D, Plantone F, Iorio R (2013) Teaching NeuroImages: basal ganglia involvement in facio-brachial dystonic seizures associated with LGI1 antibodies. Neurology 80:183–184
Boesebeck F, Schwarz O, Dohmen B, Graef U, Vestring T, Kramme C, Bien CG (2013) Faciobrachial dystonic seizures arise from cortico-subcortical abnormal brain areas. J Neurol 260:1684–1686
Fidzinski P, Jarius S, Gaebler C, Boegner F, Nohr R, Ruprecht K (2014) Faciobrachial dystonic seizures and antibodies to Lgi1 in a 92-year-old patient: a case report. J Neurol Sci 347:404–405
Andrade D, Tai P, Dalmau J, Wennberg R (2011) Tonic seizures: a diagnostic clue of anti-LGI1 encephalitis? Neurology 77:2140–2143
Lancaster E, Huijbers MG, Bar V, Boronat A, Wong A, Martinez-Hernandez E, Wilson C, Jacobs D, Lai M, Walker RW, Graus F, Bataller L, Illa I, Markx S, Strauss KA, Peles E, Scherer SS, Dalmau J (2011) Investigations of Caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol 69:303–311
Kotsenas AL, Watson RE, Pittock SJ, Britton JW, Hoye SL, Quek AML, Shin C, Klein CJ (2014) MRI findings in autoimmune voltage-gated potassium channel complex encephalitis with seizures: one potential etiology for mesial temporal sclerosis. Am J Neuroradiol 35:84–89
Szots M, Marton A, Kover F, Kiss T, Berki T, Nagy F, Illes Z (2014) Natural course of LGI1 encephalitis: 3–5 years of follow-up without immunotherapy. J Neurol Sci 343:198–202
Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, Zuliani L, Watanabe O, Lang B, Buckley C, Vincent A (2012) Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol 72:241–255
Iorio R, Lennon VA (2012) Neural antigen-specific autoimmune disorders. Immunol Rev 248:104–121
Wellmer J, Quesada CM, Rothe L, Elger CE, Bien CG, Urbach H (2013) Proposal for a magnetic resonance imaging protocol for the detection of epileptogenic lesions at early outpatient stages. Epilepsia 44:1977–1987
Urbach H, Mast H, Egger K, Mader I (2015) Presurgical MR imaging in epilepsy. Clin Neuroradiol
Hacohen Y, Absoud M, Woodhall M, Cummins C, De Goede CG, Hemingway C, Jardine PE, Kneen R, Pike MG, Whitehouse WP, Wassmer E, Waters P, Vincent A, Lim M, UK & Ireland Childhood CNS Inflammatory Demyelination Working Group (2014) Autoantibody biomarkers in childhood-acquired demyelinating syndromes: results from a national surveillance cohort. J Neurol Neurosurg Psychiatry 85:456–461
Silva J, Sharma S, Hughes B, Yu YE, Cowell JK (2010) Homozygous inactivation of the LGI1 gene results in hypomyelination in the peripheral and central nervous systems. J Neurosci Res 88:3328–3336
Faivre-Sarrailh C, Devaux JJ (2013) Neuro-glial interactions at the nodes of Ranvier: implication in health and diseases. Front Cell Neurosci 7:196
Buckley C, Oger J, Clover L, Tüzün E, Carpenter K, Jackson M, Vincent A (2001) Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 50:73–78
Schott JM, Harkness K, Barnes J, della Rocchetta AI, Vincent A, Rossor MN (2003) Amnesia, cerebral atrophy, and autoimmunity. Lancet 361:1266
Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, Clover L, Parkinson A, Bien CG, Omer S, Lang B, Rossor MN, Palace J (2004) Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 127:701–712
Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S (2004) Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology 62:1177–1182
Kröll-Seger J, Bien CG, Huppertz H (2009) Non-paraneoplastic limbic encephalitis associated with antibodies to potassium channels leading to bilateral hippocampal sclerosis in a pre-pubertal girl. Epileptic Disord 11:54–59
Baumgartner A, Rauer S, Mader I, Meyer PT (2013) Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol 260:2744–2753
Shin YW, Lee ST, Shin JW, Moon J, Lim JA, Byun JI, Kim TJ, Lee KJ, Kim YS, Park KI, Jung KH, Lee SK, Chu K (2013) VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 265:75–81
Wegner F, Wilke F, Raab P, Tayeb SB, Boeck A, Haense C, Trebst C, Voss E, Schrader C, Logemann F, Ahrens J, Leffler A, Rodriguez-Raecke R, Dengler R, Geworski L, Bengel FM, Berding G, Stangel M, Nabavi E (2014) Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-d-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F-fluoro-2-deoxy-d-glucose positron emission tomography. BMC Neurol 14:136
Acknowledgments
The authors thank Dieter Hauschke and Lioudmila Bogatyreva, Center for Medical Biometry and Medical Informatics, University of Freiburg, for statistical consulting.
Ethical standards and patient consent
We declare that this study has been approved by the Institutional Review Board and has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki and its later amendments. Due to the retrospective nature of this study, informed consent was waived.
Conflict of interest
SR receives consulting and lecture fees, grant, and research support from Bayer Vital GmbH, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis, Baxter, RG, and Teva. SR is a founding executive board member of ravo Diagnostika GmbH. MPM received payments for lectures, manuscript preparation, and travel expenses from EISAI and UCB. JL received honoraria for lectures sponsored by Euroimmun. HH has participated in meetings sponsored by or received honoraria for lectures from Bayer Schering, Biogen Idec, Merck Serono, and Novartis. CGB gave scientific advice to Eisai (Frankfurt, Germany) and UCB (Monheim, Germany); undertook industry-funded travel with support of Eisai (Frankfurt, Germany), UCB (Monheim, Germany), Desitin (Hamburg, Germany), and Grifols (Frankfurt, Germany); obtained honoraria for speaking engagements from Eisai (Frankfurt, Germany), UCB (Monheim, Germany), Desitin (Hamburg, Germany), diamed (Köln, Germany), and Fresenius Medical Care (Bad Homburg, Germany); and received research support from Astellas Pharma (München, Germany), Octapharma (Langenfeld, Germany), diamed (Köln, Germany), and Fresenius Medical Care (Bad Homburg, Germany). CGB’s employer (Krankenhaus Mara, Bielefeld, Germany) runs a laboratory for the detection of auto-antibodies including those described in this study; external senders are charged for antibody diagnostics. AB has received consulting and lecture fees, grant, and research support from Bayer Vital GmbH, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis, and Teva.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urbach, H., Rauer, S., Mader, I. et al. Supratentorial white matter blurring associated with voltage-gated potassium channel-complex limbic encephalitis. Neuroradiology 57, 1203–1209 (2015). https://doi.org/10.1007/s00234-015-1581-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1581-x