Abstract
Introduction
The aim of this study was to evaluate whether normal controls and human immunodeficiency virus (HIV) patients with and without planning deficits differ on white matter integrity.
Methods
A total of 34 HIV-positive patients with planning deficits were compared with 13 HIV-positive patients without planning deficits and 19 gender-, age-, and education-matched control subjects. Diffusion tensor imaging (DTI) was performed along 30 noncolinear directions in a 1.5-T scanner. For tract-based spatial statistics analysis, a white matter skeleton was created, and a permutation-based inference with 5000 permutations with a threshold of p < 0.05 was used to identify abnormalities in fractional anisotropy (FA). The median, radial, and axial diffusivities were also projected onto the mean FA skeleton.
Results
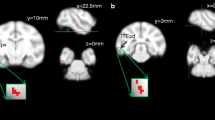
Compared with controls, HIV-positive patients with planning deficits had decreased FA in bilateral anterior thalamic radiations, bilateral inferior fronto-occiptal fasciculi, genu and splenium of the corpus callosum, bilateral superior longitudinal fascicule, and bilateral uncinate fasciculi. Compared to HIV-positive patients without planning deficits, patients with planning deficits had decreased FA in bilateral anterior thalamic radiations, bilateral inferior fronto-occiptal fasciculi, genu of the corpus callosum, bilateral superior longitudinal fascicule, and right uncinate fascicule.
Conclusion
DTI can detect extensive white matter abnormalities in the normal-appearing white matter of HIV-positive patients with planning deficits compared with controls and HIV-positive patients without planning deficits.


Similar content being viewed by others
References
Karampekios S, John Hesselink J (2005) Cerebral infections. Eur Radiol 15:485–493
Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R (2004) Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 46(5):339–350
Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC (2005) Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. AJNR Am J Neuroradiol 26:2275–2281
Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W (2001) Diffusion tensor imaging of HIV patients and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol 22:277–83
Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB (2006) Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol 27:656–60
Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO (2001) White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res 106(1):15–24
Ragin AB, Storey P, Cohen BA, Epstein LG, Edelman RR (2004) Whole brain diffusion tensor imaging in HIV-associated cognitive impairment. AJNR Am J Neuroradiol 25(2):195–200
Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG (2005) Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol 11:292–298
Leite SC, Corrêa DG, Doring TM et al (2013) Diffusion tensor MRI evaluation of the corona radiata, cingulate gyri, and corpus callosum in HIV patients. J Magn Reson Imaging 38(6):1488–1493
Gongvatana A, Schweinsburg BC, Taylor MJ et al (2009) White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol 15(2):187–95
Chen Y, An H, Zun H et al (2009) White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. NeuroImage 47:1154–1162
Stubbe-Dräger B, Deppe M, Mohammadi S et al (2012) Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurol 12:23
Antinori A, Arendt G, Becker JT et al (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799
Elliott R (2003) Executive functions and their disorders. Br Med Bull 65:49–59
Jurado MB, Rosselli M (2007) The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev 17:213–233
Cattie JE, Doyle K, Weber E et al (2012) Planning deficits in HIV-associated neurocognitive disorders: component processes, cognitive correlates, and implications for everyday functioning. J Clin Exp Neuropsychol 34(9):906–918
Reger M, Welsh R, Razani J, Martin DJ, Boone KB (2002) A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 8:410–424
Dawes S, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre S (2008) Variable patterns of neuropsychological performance in HIV-1 infection. J Clin Exp Neuropsychol 30:613–626
Woods SP, Moore DJ, Weber E, Grant I (2009) Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 19:152–168
Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G (1993) Wisconsin card sorting test manual (revised and expanded). Psychological Assessment Resources, Odessa
Cunha JA, Trentini CM, Argimon I, Oliveira MS, Werlang BG, Prieb RGG (2005) Adaptação e padronização brasileira do Manual do Teste Wisconsin de Classificação de Cartas. Casa do Psicólogo
Kavé G, Heled E, Vakil E, Agranov E (2011) Which verbal fluency measure is most useful in demonstrating executive deficits after traumatic brain injury? J Clin Exp Neuropsychol 33(3):358–365
Gauthier L, Dehaut F, Joanette Y (1989) The bells test: a quantitative and qualitative test for visual neglect. J Int Neuropsychol Soc 11:49–54
Fonseca RP, Oliveira C, Gindri G, Zimmermann N, Reppold C (2010) Teste Hayling: um instrumento de avaliação de componentes das funções executivas. In: Hutz C (ed) Avaliação psicológica e neuropsicológica de crianças e adolescente. Casa do Psicólogo, São Paulo, pp 337–364
War Department Adjutant General′s Office (1944) Army individual test battery: manual of directions and scoring. Edited by: Washington DC: War Department Adjutant General′s Office
Wechsler D (1997) Wechsler adult intelligence scale—III. The Psychological Corporation, San Antonio
Salgado JV, Malloy-Diniz LF, Abrantes SSC et al (2011) Applicability of the rey auditory-verbal learning test to an adult sample in Brazil. Rev Bras Psiquiatr 33:234–237
Fonseca RP, Salles JF, Parente MAMP (2009) Instrumento de avaliação neuropsicológica breve NEUPSILIN. Vetor, São Paulo
Fonseca RP, Parente MAMP, Côté H, Ska B, Joanette Y (2008) Bateria montreal de avaliação da comunicação—Bateria MAC. Pró-Fono, São Paulo
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:208–219
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Kramer JH, Mungas D, Possin KL et al (2014) NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc 20:11–19
Gouveia PAR, Brucki SMD, Malheiros SMF, Bueno OFA (2007) Disorders in planning and strategy application in frontal lobe lesion patients. Brain Cogn 63:240–246
Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S (2007) Regional frontal injuries cause distinct impairments in cognitive control. Neurology 68:1515–1523
Grafman J (1995) Similarities and distinctions among current models of prefrontal cortical functions. Ann N Y Acad Sci 769:337–368
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55:302–308
Smith AB, Smirniotopoulos JG, Rushing EJ (2008) From the archives of the AFIP—central nervous system infections associated with human immunodeficiency virus infection: radiologic–pathologic correlation. RadioGraphics 28:2033–2058
Ethical standards and patient consent
We declare that all human and animals studies have been approved by the Ethical Review Board of the Clementino Fraga Filho University Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corrêa, D.G., Zimmermann, N., Doring, T.M. et al. Diffusion tensor MR imaging of white matter integrity in HIV-positive patients with planning deficit. Neuroradiology 57, 475–482 (2015). https://doi.org/10.1007/s00234-015-1489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1489-5