Abstract
Introduction
The objectives of this study were to study the age-specific activation patterns of cerebral areas during motor execution (ME) and motor imaging (MI) of the upper extremities and to discuss the age-related neural mechanisms associated with ME or MI.
Methods
The functional magnetic resonance imaging technique was used to monitor the pattern and intensity of brain activation during the ME and MI of the upper extremities in 20 elderly (>50 years) and 19 young healthy subjects (<25 years).
Results
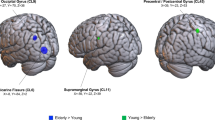
No major differences were identified regarding the activated brain areas during ME or MI between the two groups; however, a minor difference was noted. The intensity of the activated brain area during ME was stronger in the older group than in the younger group, while the results with MI were the opposite. The posterior central gyrus and supplementary motor area during MI were more active in the younger group than in the older group. The putamen, lingual, and so on demonstrated stronger activation during dominant hand MI in the older group.
Conclusion
The results of this study revealed that the brain structure was altered and that neuronal activity was attenuated with age, and the cerebral cortex and subcortical tissues were found to be over-activated to achieve the same level of ME and MI, indicating that the activating effects of the left hemisphere enhanced with age, whereas the inhibitory effects declined during ME, and activation of the right hemisphere became more difficult during MI.






Similar content being viewed by others
References
Crosbie JH, McDonough SM, Gilmore DH (2004) The adjunctive role of mental practice in the rehabilitation of the upper limb after hemiplegic stroke: a pilot study. Clin Rehabil 18:60–68
Jackson PL, Doyon J, Richards CL (2004) The efficacy of combined physical and mental practice in the learning of a foot-sequence task after stroke: a case report. Neurorehabil Neural Repair 18:106–111
Page SJ, Levine P, Leonard A (2007) Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke 38:1293–1297
Johnson-Frey SH (2004) Stimulation through simulation? Motor imagery and functional reorganization in hemiplegic stroke patients. Brain Cogn 55:328–331
Malouin F, Richards CL, Doyon J (2004) Training mobility tasks after stroke with combined mental and physical practice: a feasibility study. Neurorehabil Neural Repair 18:66–75
Crammond DJ (1997) Motor imagery: never in your wildest dream. Trends Neurosci 20:54–57
Jeannerod M (1994) The representing brain: neural correlates of motor intention and imagery. Behav Brain Sci 17:187–245
Johnson SH, Rotte M, Grafton ST (2002) Selective activation of a parietofrontal circuit during implicitly imagined prehension. Neuro Image 17:1693–1704
Johnson SH, Rotte M, Grafton ST (1999) Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci 11:491–501
Gerardin E, Sirigu A, Lehéricy A (2000) Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex 10:1092–1096
Jeannerod M (2001) Neural simulation of action: a unifying mechanism for motor cognition. Neuro Image 14:S103–S109
Kosslyn SM, Ganis G, Thompson WL (2001) Neural foundations of imagery. Nat Rev Neurosci 2:635–642
Cumming J, Hall C (2002) Athletes’ use of imagery in the off-season. Sport Psychol 16:160e172
Gregg M, Hall C, Nederhof E (2005) The imagery ability, imagery use and performance relationship. Sport Psychol 19:93–99
Gregg M, Hall C, McGowan E (2011) The relationship between imagery ability and imagery use among athletes. J Appl Sport Psychol 23:129e141
Hall C, Mack D, Paivio A (1998) Imagery use by athletes: development of the sport imagery questionnaire. Int J Sport Psychol 29:73–89
Decety J (1996) Do imagined and executed actions share the same neural substrate? Brain Res Cogn Brain Res 3:87–93
Naito E, Kochiyama T, Kitada R (2002) Internally simulated movement sensations during motor imagery activate cortical motor areas and the cerebellum. J Neurosci 22:3683–3691
Johnson SH, Spreh G, Saykin AJ (2002) Intact motor imagery in chronic upper limb hemiplegics: evidence for activity independent action representations. J Cogn Neurosci 14:841–852
Wenk GL, Pierce DJ, Struble RG (1989) Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging 10:11–19
Volkow ND, Gur RC, Wang GJ (1998) Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155:344–349
Calautti C, Serrati C, Baron JC (2001) Effects of age on brain activation during auditory-cued thumb-to-index opposition: a positron emission tomography study. Stroke 32:139–146
Ward NS, Swayne OB, Newton JM (2008) Age-dependent changes in the neural correlates of force modulation: an fMRI study. Neurobiol Aging 29:1434–1446
Mattay VS, Fera F, Tessitore A (2002) Neurophysiological correlates of age-related changes in human motor function. Neurology 58:630–635
Ward NS, Frackowiak RS (2003) Age-related changes in the neural correlates of motor performance. Brain 126:873–888
Nair DG, Purcott KL, Fuchs A (2003) Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: a functional MRI study. Brain Res Cogn Brain Res 15:250–260
Stephan KM, Fink GR, Passingham RE (1995) Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73:373–386
Szameitat AJ, Shen S, Conforto A (2002) Cortical activation during executed, imagined, observed, and passive wrist movements in healthy volunteers and stroke patients. Neuroimage 62:266–280
Hutchinson S, Kobayashi M, Horkan CM (2002) Age-related differences in movement representation. Neuroimage 17:1720–1728
Zapparoli L, Invernizzi P, Gandola M (2013) Mental images across the adult lifespan: a behavioural and fMRI investigation of motor execution and motor imagery. Exp Brain Res 224:519–540
Ehrsson HH, Geyer S, Naito E (2003) Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol 90:3304–3316
Macuga KL, Frey SH (2012) Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage 59:2798–2807
Haaland KY, Harrington DL (1994) Limb-sequencing deficits after left but not right hemisphere damage. Brain Cogn 24:104–122
Small SL, Hlustik P, Noll DC (2002) Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain 125:1544–1557
Acknowledgment
This work was supported by 2012 National Nature Science “Study on the neural mechanism of motor imagery in the rehabilitation of patients with stroke hemiplegia” (No. 81171866).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Qiu, M., Liu, C. et al. Age-specific activation of cerebral areas in motor imagery—a fMRI study. Neuroradiology 56, 339–348 (2014). https://doi.org/10.1007/s00234-014-1331-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1331-5