Abstract
Introduction
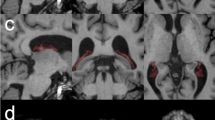
The cerebrospinal fluid (CSF) plays a major role in the physiology of the central nervous system. The continuous turnover of CSF is mainly attributed to the highly vascularized choroid plexus (CP) located in the cerebral ventricles which represent a complex interface between blood and CSF. We propose a method for evaluating CP functionality in vivo using perfusion MR imaging and establish the age-related changes of associated parameters.
Methods
Fifteen patients with small intracranial tumors were retrospectively studied. MR Imaging was performed on a 3T MR Scanner. Gradient-echo echo planar images were acquired after bolus injection of gadolinium-based contrast agent (CA). The software developed used the combined T1- and T2-effects. The decomposition of the relaxivity signals enables the calculation of the CP capillary permeability (K2). The relative cerebral blood volume (rCBV), mean transit time (MTT), and signal slope decrease (SSD) were also calculated.
Results
The mean permeability K2 of the extracted CP was 0.033+/−0.18 s-1. K2 and SSD significantly decreased with subject’s age whereas MTT significantly increased with subject’s age. No significant correlation was found for age-related changes in rCBV and rCBF.
Conclusion
The decrease in CP permeability is in line with the age-related changes in CSF secretion observed in animals. The MTT increase indicates significant structural changes corroborated by microscopy studies in animals or humans. Overall, DSC MR-perfusion enables an in vivo evaluation of the hemodynamic state of CP. Clinical applications such as neurodegenerative diseases could be considered thanks to specific functional studies of CP.




Similar content being viewed by others
References
Czosnyka Z, Owler B, Keong N, Santarius T, Baledent O, Pickard JD, Czosnyka M (2011) Impact of duration of symptoms on CSF dynamics in idiopathic normal pressure hydrocephalus. Acta Neurol Scand 123:414–418
Serot JM, Peltier J, Fichten A, Ledeme N, Bourgeois AM, Jouanny P, Toussaint P, Legars D, Godefroy O, Maziere JC (2011) Reduced CSF turnover and decreased ventricular Abeta42 levels are related. BMC Neurosci 12:42
Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, Rubenstein E, Possin K, Saul TA (2001) The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 57:1763–1766
Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D (2003) Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol 2:506–511
Redzic ZB, Segal MB (2004) The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev 56:1695–1716
Faraci FM, Mayhan WG, Williams JK, Heistad DD (1988) Effects of vasoactive stimuli on blood flow to choroid plexus. Am J Physiol 254:H286–H291
Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J (2005) The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol 71:1–52
Tauc M, Vignon X, Bouchaud C (1984) Evidence for the effectiveness of the blood–CSF barrier in the fetal rat choroid plexus. A freeze-fracture and peroxidase diffusion study. Tissue Cell 16:65–74
Zheng W, Blaner WS, Zhao Q (1999) Inhibition by lead of production and secretion of transthyretin in the choroid plexus: its relation to thyroxine transport at blood-CSF barrier. Toxicol Appl Pharmacol 155:24–31
Deane R, Segal MB (1985) The transport of sugars across the perfused choroid plexus of the sheep. J Physiol 362:245–260
Chen RL, Kassem NA, Redzic ZB, Chen CP, Segal MB, Preston JE (2009) Age-related changes in choroid plexus and blood–cerebrospinal fluid barrier function in the sheep. Exp Gerontol 44:289–296
May C, Kaye JA, Atack JR, Schapiro MB, Friedland RP, Rapoport SI (1990) Cerebrospinal fluid production is reduced in healthy aging. Neurology 40:500–503
Gideon P, Thomsen C, Stahlberg F, Henriksen O (1994) Cerebrospinal fluid production and dynamics in normal aging: a MRI phase-mapping study. Acta Neurol Scand 89:362–366
Serot JM, Bene MC, Faure GC (2003) Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci 8:515–521
Serot JM, Bene MC, Foliguet B, Faure GC (2000) Morphological alterations of the choroid plexus in late-onset Alzheimer’s disease. Acta Neuropathol 99:105–108
Bouzerar R, Chaarani B, Pottie O, Gondry-Jouet C, Zmudka J, Serot JM, Baledent O (2012) Imaging of the choroid plexus using perfusion MR imaging: is it possible? Paper presented at the ISMRM 20th Annual Meeting, Melbourne, Australia, 5–11 May
Sorensen AG, Tievsky AL, Ostergaard L, Weisskoff RM, Rosen BR (1997) Contrast agents in functional MR imaging. J Magn Reson Imaging 7:47–55
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Donahue KM, Krouwer HG, Rand SD, Pathak AP, Marszalkowski CS, Censky SC, Prost RW (2000) Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med 43:845–853
Benner T, Heiland S, Erb G, Forsting M, Sartor K (1997) Accuracy of gamma-variate fits to concentration-time curves from dynamic susceptibility-contrast enhanced MRI: influence of time resolution, maximal signal drop and signal-to-noise. Magn Reson Imaging 15:307–317
Knopp EA, Cha S, Johnson G, Mazumdar A, Golfinos JG, Zagzag D, Miller DC, Kelly PJ, Kricheff II (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211:791–798
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (eds) (2007) Numerical recipes: the art of scientific computing. Third Edition edn. Cambridge University Press, New-York
Yeom KW, Mitchell LA, Lober RM, Barnes PD, Vogel H, Fisher PG, Edwards MS (2013) Arterial spin-labeled perfusion of pediatric brain tumors. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A3670
Zimny A, Sasiadek M (2011) Contribution of perfusion-weighted magnetic resonance imaging in the differentiation of meningiomas and other extra-axial tumors: case reports and literature review. J Neurooncol 103:777–783
Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB (2009) Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp 30:1120–1132
Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT (2007) Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage 37:430–439
Asllani I, Habeck C, Borogovac A, Brown TR, Brickman AM, Stern Y (2009) Separating function from structure in perfusion imaging of the aging brain. Hum Brain Mapp 30:2927–2935
Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS (1991) Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11:684–689
Stoquart-ElSankari S, Baledent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME (2007) Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab 27:1563–1572
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36
Williams DS, Detre JA, Leigh JS, Koretsky AP (1992) Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA 89:212–216
Carr JP, Buckley DL, Tessier J, Parker GJ (2007) What levels of precision are achievable for quantification of perfusion and capillary permeability surface area product using ASL? Magn Reson Med 58:281–289
van Gelderen P, de Zwart JA, Duyn JH (2008) Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med 59:788–795
Rmeily-Haddad M, Baledent O, Stoquart El Sankari S, Serot JM, Bailly P, Meyer ME (2011) The kinetics of 18f-fluorodeoxyglucose uptake in the choroid plexus. Int J Imaging Syst Technol 21:107–114
Farrall AJ, Wardlaw JM (2009) Blood-brain barrier: ageing and microvascular disease—systematic review and meta-analysis. Neurobiol Aging 30:337–352
Shu Y, Liu M, Chen S, Chen X, Wang J (2011) New insight into molecular interactions of imidazolium ionic liquids with bovine serum albumin. J Phys Chem B 115:12306–12314
Serot JM, Foliguet B, Bene MC, Faure GC (2001) Choroid plexus and ageing in rats: a morphometric and ultrastructural study. Eur J Neurosci 14:794–798
Masseguin C, LePanse S, Corman B, Verbavatz JM, Gabrion J (2005) Aging affects choroidal proteins involved in CSF production in Sprague-Dawley rats. Neurobiol Aging 26:917–927
Gerrits PO, Kortekaas R, de Weerd H, Luiten PG, van der Want JJ, Veening JG (2013) Spumiform basement membrane aberrations in the microvasculature of the midbrain periaqueductal gray region in hamster: rostro-caudal pathogenesis? Neuroscience 228:128–138
Gerrits PO, Kortekaas R, de Weerd H, Veenstra-Algra A, Luiten PG, van der Want JJ, Veening JG (2013) Spumiform capillary basement membrane swelling: a new type of microvascular degeneration in senescent hamster. Neurobiol Aging 34:1277–1286
Preston JE (2001) Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech 52:31–37
Bersani G, Garavini A, Taddei I, Tanfani G, Pancheri P (1999) Choroid plexus calcification as a possible clue of serotonin implication in schizophrenia. Neurosci Lett 259:169–172
Farkas E, De Vos RA, Jansen Steur EN, Luiten PG (2000) Are Alzheimer’s disease, hypertension, and cerebrocapillary damage related? Neurobiol Aging 21:235–243
Ostergaard L, Aamand R, Gutierrez-Jimenez E, Ho YC, Blicher JU, Madsen SM, Nagenthiraja K, Dalby RB, Drasbek KR, Moller A, Braendgaard H, Mouridsen K, Jespersen SN, Jensen MS, West MJ (2013) The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol Aging 34:1018–1031
Acknowledgments
MRI operators at University Hospital, Amiens, France, are gratefully acknowledged for their technical contribution.
Conflict of Interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouzerar, R., Chaarani, B., Gondry-Jouet, C. et al. Measurement of choroid plexus perfusion using dynamic susceptibility MR imaging: capillary permeability and age-related changes. Neuroradiology 55, 1447–1454 (2013). https://doi.org/10.1007/s00234-013-1290-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-013-1290-2