Abstract
Introduction
Cognitive impairment after aneurysmal subarachnoid hemorrhage (aSAH) is frequently detected. Here, we describe the pattern of cerebral (gray matter) atrophy and its clinical relevance after treatment of aSAH caused by a ruptured anterior cerebral artery (ACA) aneurysm.
Methods
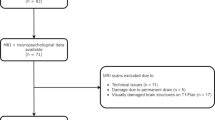
Thirty-seven aSAH patients with ACA aneurysm (17 surgical, 20 endovascular treatment) and a good or moderate clinical outcome (Glasgow Outcome Scale V or IV) and 30 controls underwent brain MRI. Voxel-based morphometric analysis was applied to compare the patients and controls. Patients also underwent a detailed neuropsychological assessment.
Results
The comparisons between controls and either all patients (n = 37) or the subgroup of surgically treated patients (n = 17) revealed bilateral cortical atrophy in the frontal lobes, mainly in the basal areas. The brainstem, bilateral thalamic and hypothalamic areas, and ipsilateral caudate nucleus were also involved. Small areas of atrophy were detected in temporal lobes. The hippocampus and parahippocampal gyrus showed atrophy ipsilateral to the surgical approach. In the subgroup of endovascularly treated patients (n = 15), small areas of atrophy were detected in the bilateral orbitofrontal cortex and in the thalamic region. Twenty patients (54%) showed cognitive deficits in neuropsychological assessment.
Conclusion
Group analysis after aSAH and treatment of the ruptured ACA aneurysm revealed gray matter atrophy, principally involving the frontobasal cortical areas and hippocampus ipsilateral to the surgical approach. Areas of reduced gray matter were more pronounced after surgical than endovascular treatment. Together with possible focal cortical infarctions and brain retraction deficits in individual patients, this finding may explain the neuropsychological disturbances commonly detected after treatment of ruptured ACA aneurysms.





Similar content being viewed by others
References
Hackett ML, Anderson CS (2000) Health outcomes 1 year after subarachnoid hemorrhage: An international population-based study. The Australian Cooperative Research on Subarachnoid Hemorrhage Study Group. Neurology 55:658–662
Fontanella M, Perozzo P, Ursone R et al (2003) Neuropsychological assessment after microsurgical clipping or endovascular treatment for anterior communicating artery aneurysm. Acta Neurochir (Wien) 145:867–872 discussion 872
Buchanan KM, Elias LJ, Goplen GB (2000) Differing perspectives on outcome after subarachnoid hemorrhage: the patient, the relative, the neurosurgeon. Neurosurgery 46:831–838 discussion 838-40
Bendel P, Koivisto T, Kononen M et al (2008) MR imaging of the brain 1 year after aneurysmal subarachnoid hemorrhage: randomized study comparing surgical with endovascular treatment. Radiology 246:543–552
Hadjivassiliou M, Tooth CL, Romanowski CA et al (2001) Aneurysmal SAH: cognitive outcome and structural damage after clipping or coiling. Neurology 56:1672–1677
Kivisaari RP, Salonen O, Servo A et al (2001) MR imaging after aneurysmal subarachnoid hemorrhage and surgery: a long-term follow-up study. AJNR Am J Neuroradiol 22:1143–1148
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. Neuroimage 11:805–821
Whitwell JL, Przybelski SA, Weigand SD et al (2007) 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain 130:1777–1786
Beyer MK, Janvin CC, Larsen JP et al (2007) A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry 78:254–259
Mezzapesa DM, Ceccarelli A, Dicuonzo F et al (2007) Whole-brain and regional brain atrophy in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 28:255–259
Spencer MD, Moorhead TW, McIntosh AM et al (2007) Grey matter correlates of early psychotic symptoms in adolescents at enhanced risk of psychosis: a voxel-based study. Neuroimage 35:1181–1191
Bendel P, Koivisto T, Hanninen T et al (2006) Subarachnoid hemorrhage is followed by temporomesial volume loss: MRI volumetric study. Neurology 67:575–582
Koivisto T, Vanninen R, Hurskainen H et al (2000) Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 31:2369–2377
Vanninen R, Koivisto T, Saari T et al (1999) Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils–a prospective randomized study. Radiology 211:325–336
Good CD, Johnsrude I, Ashburner J et al (2001) Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 14:685–700
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878
Lancaster JL, Woldorff MG, Parsons LM et al (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131
Duvernoy H, Duvernoy H (1999) The human brain surface, blood supply, and three-dimensional sectional anatomy, 2nd edn. Springer, Wien
Wechsler D (1981) Wechsler Adult Intelligence Scale—revised. The Psychological, New York
HD LMD, Loring DW (2004) Neuropsychological assessment. Oxford University Press, New York
Wechsler D (1974) Wechsler memory scale manual. The Psychological, San Antonio
Reitan R (1958) Validity of the trail making tests as an indicator of organic brain damage. Percept Mot Skills 8:271–276
Golden C (1978) Stroop color and word tests. Stoerting, Chicago
Egge A, Waterloo K, Sjoholm H et al (2005) Outcome 1 year after aneurysmal subarachnoid hemorrhage: relation between cognitive performance and neuroimaging. Acta Neurol Scand 112:76–80
Hutter BO, Gilsbach JM (1993) Which neuropsychological deficits are hidden behind a good outcome (Glasgow = I) after aneurysmal subarachnoid hemorrhage? Neurosurgery 33:999–1005 discussion 1005-6
Hutter BO, Gilsbach JM (1992) Cognitive deficits after rupture and early repair of anterior communicating artery aneurysms. Acta Neurochir (Wien) 116:6–13
Mavaddat N, Kirkpatrick PJ, Rogers RD et al (2000) Deficits in decision-making in patients with aneurysms of the anterior communicating artery. Brain 123(Pt 10):2109–2117
Mayer SA, Kreiter KT, Copeland D et al (2002) Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology 59:1750–1758
Vilkki J, Holst P, Ohman J et al (1989) Cognitive deficits related to computed tomographic findings after surgery for a ruptured intracranial aneurysm. Neurosurgery 25:166–172
Miller EK (2000) The prefrontal cortex and cognitive control. Nat Rev Neurosci 1:59–65
Frazer D, Ahuja A, Watkins L et al (2007) Coiling versus clipping for the treatment of aneurysmal subarachnoid hemorrhage: a longitudinal investigation into cognitive outcome. Neurosurgery 60:434–441 discussion 441-2
Bellebaum C, Schafers L, Schoch B et al (2004) Clipping versus coiling: neuropsychological follow up after aneurysmal subarachnoid haemorrhage (SAH). J Clin Exp Neuropsychol 26:1081–1092
Molyneux AJ, Kerr RS, Yu LM et al (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366:809–817
Romner B, Sonesson B, Ljunggren B et al (1989) Late magnetic resonance imaging related to neurobehavioral functioning after aneurysmal subarachnoid hemorrhage. Neurosurgery 25:390–396 discussion 396-7
Wermer MJ, Donswijk M, Greebe P et al (2007) Anosmia after aneurysmal subarachnoid hemorrhage. Neurosurgery 61:918–922 discussion 922-3
Moman MR, Verweij BH, Buwalda J et al (2008) Anosmia after endovascular and open surgical treatment of intracranial aneurysms. J Neurosurg 110:482–486
Kumar N (2007) Superficial siderosis: associations and therapeutic implications. Arch Neurol 64:491–496
Vymazal J, Urgosik D, Bulte JW (2000) Differentiation between hemosiderin- and ferritin-bound brain iron using nuclear magnetic resonance and magnetic resonance imaging. Cell Mol Biol (Noisy-le-grand) 46:835–842
Acknowledgments
The authors are grateful to the neuropsychologist Heleena Hurskainen who has performed all the neuropsychological evaluations. This study has been supported by the Kuopio University Hospital, grant 5063515, and Finnish Society of Radiology and Instrumentarium Foundation.
Conflict of interest statement
We declare that we have no conflict of interest.
Author contributions
The guarantors of integrity of entire study are Paula Bendel and Ritva Vanninen. Study concepts, design, and definition of intellectual concepts were done by P. Bendel, T. Koivisto, and R. Vanninen. Literature research was completed by P. Bendel. The study design and data analysis/interpretation and manuscript editing were carried out by all authors. Clinical studies were performed by P. Bendel T. Koivisto, M. Äikiä, T. Hänninen, and R. Vanninen. All authors took part in the data acquisition and data analysis. P. Bendel, E. Niskanen, and M. Könönen worked on the statistical analysis. All authors arranged the manuscript preparation and editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bendel, P., Koivisto, T., Niskanen, E. et al. Brain atrophy and neuropsychological outcome after treatment of ruptured anterior cerebral artery aneurysms: a voxel-based morphometric study. Neuroradiology 51, 711–722 (2009). https://doi.org/10.1007/s00234-009-0552-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-009-0552-5