Abstract
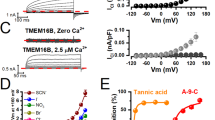
TMEM16A is the molecular basis of calcium-activated chloride channels and shows Ca2+-dependent gating. It is critical to understand how the Ca2+ sensors dynamically control the gate of TMEM16A. However, the detailed mechanism by which the calcium ions bind and open the channel is still obscure. In this study, the authors confirmed that there are two Ca2+ sensors which cooperatively couple together in TMEM16A. Our data show that mutations at both Ca2+-sensitive domains, E447Y and E702Q-E705Q, weaken the Ca2+ affinity for TMEM16A channel. The EC50 for WT, E447Y, and E702Q-E705Q are 0.53 ± 0.11, 14.5 ± 0.3, and 26.5 ± 3.6 μM, respectively. The triple mutation, including both of the Ca2+ sensors, E447Y-E702Q-E705Q, with EC50 as 55.6 ± 5.1 μM, results in much further right-shifted dose response curve than the single sensor’s mutations (E447Y, E702Q-E705Q) do, which indicates that there is a cooperation between the two Ca2+-sensitive domains. We also found that the divalent cations, both Ca2+ and Sr2+, share common mechanism of gating the TMEM16A.





Similar content being viewed by others
References
Barish ME (1983) A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol 342:309–325
Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R (2014) X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516:207–212
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322:590–594
Chen Y, An H, Li T, Liu Y, Gao C, Guo P, Zhan Y (2011) Direct or indirect regulation of calcium-activated chloride channel by calcium. J Membr Biol 240:121–129
Hartzell C, Putzier I, Arreola J (2005) Calcium-activated chloride channels. Annu Rev Physiol 67:719–758
Huang F, Wong X, Jan LY (2012) International Union of basic and clinical pharmacology. LXXXV: calcium-activated chloride channels. Pharmacol Rev 64:1–15
Miledi R (1982) A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci 215:491–497
Pang CL (2013) Mechanism of calcium binding to TMEM16A channel via electrostatic interactions. Dissertation, Hebei University of Technology
Pang C, Cao T, Li J, Jia M, Zhang S, Ren S, An H, Zhan Y (2013) Combining fragment homology modeling with molecular dynamics aims at prediction of Ca2+ binding sites in CaBPs. J Comput Aided Mol Des 27:697–705
Tien J, Peters CJ, Wong XM, Cheng T, Jan YN, Jan LY, Yang HH (2014) A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. Elife 3:e02772
Xia XM, Zeng X, Lingle CJ (2002) Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature 418:880–884
Xiao Q, Cui Y (2014) Acidic amino acids in the first intracellular loop contribute to voltage- and calcium-dependent gating of anoctamin1/TMEM16A. PLoS ONE 9:e99376
Xiao Q, Yu K, Perez-Cornejo P, Cui Y, Arreola J, Hartzell HC (2011) Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc Natl Acad Sci USA 108:8891–8896
Yang YD, Cho H, Koo JY, TakMH Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455:1210–1215
Yu K, Duran C, Qu Z, Cui YY, Hartzell HC (2012) Explaining calcium-dependent gating of anoctamin-1 chloride channels requires a revised topology. Circ Res 110:990–999
Yuan HB (2012) Molecular mechanisms of calcium-dependent gating in calcium-activated chloride channels. Dissertation, Hebei University of Technology
Yuan H, Gao C, Chen Y, Jia M, Geng J, Zhang H, Zhan Y, Boland LM, An H (2013) Divalent cations modulate TMEM16A calcium-activated chloride channels by a common mechanism. J Membr Biol 246:893–902
Zeng XH, Xia XM, Lingle CJ (2005) Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J Gen Physiol 125:273–286
Zhang G, Yang H, Liang H, Yang J, Shi J, McFarland K, Chen Y, Cui J (2014a) A charged residue in S4 regulates coupling among the activation gate, voltage, and Ca2+ sensors in BK channels. J Neurosci 34:12280–12288
Zhang S, Chen Y, An H, Liu H, Li J, Pang C, Ji Q, Zhan Y (2014b) A novel biophysical model on calcium and voltage dual dependent gating of calcium-activated chloride channel. J Theor Biol 355:229–235
Acknowledgments
This work was supported by the Natural Science Fund for Distinguished Young Scholars of the Hebei Province of China (Grant No. C2015202340 to HA), the Fund for Outstanding Talents of Hebei Province of China (Grant No. C201400305 to HA), the Natural Science Fund of Hebei Province (Grant No. C2013202244 to YC), and the National Natural Science Fund of China (Grant No. 11247010 to HA, 11175055 to YZ, 11347017 to SZ, 31400711 to YC).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Han, Y., Zhang, S., Ren, S. et al. Two Ca2+-Binding Sites Cooperatively Couple Together in TMEM16A Channel. J Membrane Biol 249, 57–63 (2016). https://doi.org/10.1007/s00232-015-9846-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-015-9846-1