Abstract
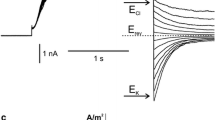
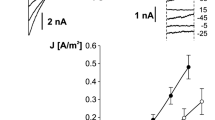
Voltage-dependent activation of slow vacuolar (SV) channels has been studied on isolated patches from red beet (Beta vulgaris L.) vacuoles. Isoosmotic variation of vacuolar K+ from 10 to 400 mM in Ca2+-free solutions at the vacuolar side shifted the SV channel activation threshold to more positive voltages. The effect of K+ could be mimicked by additions of choline or N-methyl D-glucamine and could be explained by unspecific screening of the negative surface charge. Fitting the dependence of voltage shift on K+ concentration to the Gouy-Chapman model yields a surface charge density of 0.36 ± 0.05 e−/nm2. Negative surface potential also tended to increase the local concentration of permeable ions (K+), resulting in anomalously high single-channel conductance, ∼200 pS in 10 mM KCl. An increase of ionic strength due to addition of impermeable cations greatly reduced the unitary conductance. Large positive shift of the SV channel voltage dependence, caused by physiological (0.5 mM) free vacuolar Ca2+, was partly ameliorated by increasing luminal K+. We interpreted these results as follows: K+ induced a reduction of surface potential, hence i) causing a positive shift of the voltage dependence and ii) a dilution of Ca2+ in the membrane vicinity, thus reducing the inhibitory effect of vacuolar Ca2+ and causing a negative shift of the SV channel voltage dependence, with a sum of the two shifts being negative.







Similar content being viewed by others
Notes
Ca2+ affects/reduces the surface potential in two ways: i) screening by bulk Ca2+ ions and multivalent cations is far more potent as compared to that of monovalent ones (K+); ii) partial neutralization of the surface charge due to a specific Ca2+ binding to ionizable sites within the so-called Stern layer, with the affinity for Ca2+ being 101 to 102 stronger as compared to alkali metal cations (Latorre et al., 1992; Hille, 2001). However, at a Ca2+ concentration of 0.5 mM, its effects on the surface potential are relatively small. As an example, in frog node of Ranvier, Ca2+ as a sole cation present, at 0.5 mM concentration reduced the surface potential by only 5 mV (Fig. 20.11 in Hille, 2001). This could be considered an upper limit, because i) the surface charge density in our case is 3 times lower and ii) the effects of Ca2+ on the surface charge/potential will be less pronounced on the background of K+. Therefore, the reduction of the tonoplast surface potential by luminal K+ increase will be almost unchanged in the presence of 0.5 mM Ca2+
References
Alien G.J., Sanders D. 1997. Vacuolar ion channels of higher plants. Adv. Bot. Res. 25:217–252
Apse M.P., Sottosanto J.B., Blumwald E. 2003. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 36:229–239
Carpaneto A., Cantu A.M., Gambale F. 2001. Effects of cytoplasmic Mg2+ on slowly activating channels in isolated vacuoles of Beta vulgaris. Planta 213:457–468
Cheng N.-H., Pittman J.K., Zhu J.-K., Hirschi K. 2004. The protein kinase SOS2 Activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 279:2922–2926
Cuin T.A., Miller A.J., Laurie S.A., Leigh R.A. 2003. Potassium activities in cell compartments of salt-grown barley leaves. J. Exp. Bot. 54:657–661
Dobrovinskaya O.K., Muñiz J., Pottosin I.I. 1999. Asymmetric block of the plant vacuolar Ca2+-permeable channel by organic cations. Eur. Biophys. J. 28:552–563
Felle H 1988. Cytosolic free calcium in Riccia fluitans and Zea mays: interaction of Ca2+ and pH? Planta 176:248–255
Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch. 391:85–100
Hedrich R., Barbier-Brygoo H., Felle H., Fluegge U.I., Luettge U., Maathuis F.J.M., Marx S., Prins H.B.A., Raschke K., Schnabl H., Schroeder J.I., Struve I, Taiz L., Zeigler P. 1988. General mechanisms for solute transport across the tonoplast of plant vacuoles: A patch-clamp survey of ion channels and proton pumps. Bot. Acta 101: 7–13
Hille B. 2001. Ionic Channels of Excitable Membranes, 3rd ed. Sinauer Associates, Sunderland, MA
Ivashikina N., Hedrich R. 2005. K+ currents through SV-type vacuolar channels are sensitive to elevated luminal sodium levels. Plant J. 41:106–114
Latorre R, Labarca P., Naranjo D. 1992. Surface charge effects on ion conduction in ion channels. In: Ion Channels., B. Rudy, L.E. Iverson, editors, Methods in Enzymology vol. 207 Academic Press, K San Diego pp 471–501
Leigh R.A. 1997. Solute composition of vacuoles. Adv. Bot. Res. 25:171–194
MacRobbie E.A.C. 1995. Effects of ABA on 86Rb+ fluxes at plasmalemma and tonoplast of stomatal guard cells. Plant J. 7:835–843
MacRobbie E.A.C. 1998. Signal transduction and ion channels in guard cells. Phil. Trans. R. Soc. Lond B. 353:1475–1488
MacRobbie E.A.C. 2000. ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+ (Rb+) release. Proc. Natl. Acad. Sci USA 97:12361–12368
Pei Zh.-M., Ward J.M., Schroeder J.I. 1999. Magnesium sensitizes slow vacuolar channels to physiological cytosolic calcium and inhibits fast vacuolar channels in fava bean guard cell vacuoles. Plant Physiol. 121:977–986
Penny M.G., Bowling DJ.F. 1974. A study of potassium gradients in the epidermis of intact leaves of Commelina communis L. in relation to stomatal opening. Planta 119:17–25
Pittman J.K., Hirschi K. 2003. Don’t shoot the (second) messenger: endomembrane transporters and binding proteins modulate cytosolic Ca2+ levels. Curr. Opin. Plant Biol 6:257–262
Pottosin I.I., Tikhonova L.I., Hedrich R, Schönknecht G. 1997. Slowly activating vacuolar channels can not mediate Ca2+-induced Ca2+-release. Plant J. 12:1387–1398
Pottosin I.I., Dobrovinskaya O.K., Muniz J. 2001. Conduction of monovalent and divalent cations in the slow vacuolar channel. J. Membrane Biol 181:55–65
Pottosin I.I., Martínez-Estévez M. 2003. Regulation of the fast vacuolar channel by cytosolic and vacuolar potassium. Biophys. J. 84:977–986
Pottosin I.I., Martínez-Estévez M., Dobrovinskaya O.R., Muniz J. 2003. Potassium-selective channel in the red beet vacuolar membrane. J. Exp. Bot. 54:663–667
Pottosin LI, Martínez-Estévez M., Dobrovinskaya O.R, Muniz J., Schönknecht G. 2004. Mechanism of luminal Ca2+ and Mg2+ action on the vacuolar slowly activating channels. Planta 219:1057–1070
Schulz-Lessdorf B., Hedrich R. 1995. Protons and calcium modulate SV-type channels in the vacuolar-lysosomal compartment. Channel interaction with calmodulin inhibitors. Planta 197:655–671
Schaul O. 2002. Magnesium transport and function in plants: the tip of the iceberg. Biometals 15:309–323
Talbott L.D., Zeiger E. 1996. Central roles of potassium and sucrose in guard cell osmoregulation. Plant Physiol. 111:1051–1057
Tikhonova L.I., Pottosin I.I., Dietz K.J., Schönknecht G. 1997. Fast-activating cation channel in barley mesophyll vacuoles. Inhibition by calcium. Plant J. 11:1059–1070
Walker D.J., Leigh R.A., Miller AJ. 1996. Potassium homeostasis in vacuolate plant cells. Proc. Nail Acad. Sci. USA 93:10510–10514
Ward J.M., Schroeder J.I. 1994. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6:669–683
Acknowledgement
This study was supported by CONACyT grants 29473-N and 38181-N to Igor Pottosin. The authors are indebted to Dr. Tracey Cuin for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pottosin, I., Martínez-Estévez, M., Dobrovinskaya, O. et al. Regulation of the Slow Vacuolar Channel by Luminal Potassium: Role of Surface Charge. J Membrane Biol 205, 103–111 (2005). https://doi.org/10.1007/s00232-005-0766-3
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s00232-005-0766-3