Abstract
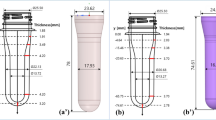
The heat transfer properties of different cooling systems dealing with Poly-Ethylene-Terephthalate (PET) bottles were investigated. The heat transfer coefficient (Ug) was measured in various fluid dynamic conditions. Cooling media were either air or water. It was shown that heat transfer coefficients are strongly affected by fluid dynamics conditions, and range from 10 W/m2 K to nearly 400 W/m2 K. PET bottle thickness effect on Ug was shown to become relevant under faster fluid dynamics regimes.









Similar content being viewed by others
Abbreviations
- A:
-
Sample A
- AS :
-
Bottle external area
- B:
-
Sample B
- C:
-
Constant
- cp :
-
Specific heat
- D:
-
Bottle external diameter
- g:
-
Gravitational acceleration
- Gr:
-
Grashof Number
- H:
-
Bottle height
- h1 :
-
Heat transfer coefficient inside the bottle
- h2 :
-
Heat transfer coefficient outside the bottle
- k:
-
PET thermal conductivity
- kw :
-
Water thermal conductivity
- L:
-
Characteristic length, volume/surface
- m:
-
Mass
- Nu:
-
Nusselt number
- Pr:
-
Prandtl number
- r1 :
-
Bottle internal radius
- r2 :
-
Bottle external radius
- Qtot :
-
Global heat flux
- Qrad :
-
Radiant heat flux
- Qconv :
-
Convective heat flux
- hrad :
-
Radiant heat transfer coefficient
- Uconv.+cond. :
-
Convective heat transfer coefficient
- Ra:
-
Rayleigh number
- Re:
-
Reynolds number
- T:
-
Temperature
- T1 :
-
Inside temperature of water
- T2 :
-
Outside temperature of cooling fluid
- T0 :
-
Initial inside temperature of water
- Tg :
-
Glass transition temperature
- Twall :
-
Bottle surface temperature
- T∞ :
-
Bulk temperature of cooling fluid
- θ:
-
Dimensionless temperature
- t:
-
Time
- Ug :
-
Global heat transfer coefficient
- α :
-
Thermal diffusivity
- α w :
-
Water thermal diffusivity
- α PET :
-
PET thermal diffusivity
- β:
-
Volumetric thermal expansion coefficient
- δ:
-
PET layer thickness
- μ:
-
Viscosity
- ν:
-
Kinematic viscosity (μ/ρ)
- ρ :
-
Density
- τ:
-
Characteristic time
References
Bischof JC, He X (2006) Thermal stability of proteins. Ann NY Acad Sci 1066:12–33
Hanschen FS et al (2012) Thermally induced degradation of aliphatic glucosinolates: identification of intermediary breakdown products and proposed degradation pathways. J Agric Food Chem 60(39):9890–9899
Jiménez-Sánchez C et al (2015) Alternatives to conventional thermal treatments in fruit-juice processing. Part 2: effect on composition, phytochemical content, and physicochemical, rheological, and organoleptic properties of fruit juices. Crit Rev Food Sci Nutr, p. posted online. ISSN:10.1080/10408398.2014.914019
Perussello CA, Viviana CM, Alvaro CDA (2011) Combined modeling of thermal properties and freezing process by convection applied to green beans. Appl Therm Eng 31:2894–2901
Berto MI, Silveira VJ (2013) Design and performance of conventional and fuzzy controls for a high temperature short time pasteurization system. J Food Process Eng 36:58–65
Bates RP, Morris JR, Crandall PG (2001) Principle and practice of small-and medium-scale fruit juice processing. FAO Agricultural Services Bulletin, Rome
Cammalleri M et al (2015) Experimental evaluation of a new thermal process for microorganisms inactivation. J Food Process Eng. doi:10.1111/jfpe.12175
Bach C et al (2013) Effect of temperature on the release of intentionally and non-intentionally added substances for poly-ethylene-terephtalate (PET) into water: chemical analysis and potential toxicity. Food Chem 139:672–680
Omari KE, Kouskou T, Guer YL (2011) Impact of shape of container on natural convection and melting inside enclosures used for passive cooling of electronic devices. Appl Therm Eng 31:3022–3035
Augusto PED, Pinheiro TF, Cristianini M (2012) Determining convective heat transfer coefficient (h) for heating and cooling of bottles in water immersion. J Food Process Eng 35:54–75
Haeri S, Shrimpton JS (2013) A correlation for the calculation of the local Nusselt number around circular cylinders in the range 10 ≤ Re ≤ 250 and 0.1 ≤ Pr ≤ 40. Int J Heat Mass Transf 59:219–229
Abu-Hijleh BA (2001) Laminar forced convection heat transfer from a cylinder covered with an orthotropic porous layer in cross-flow. J Numer Methods Heat Fluid Flow 11(2):106–120
Bird RB, Stewart WE, Lightfoot EN (2002) Transport phenomena, 2nd edn. Wiley, New York
Bernier D, Hansen GA (1972) Thermal conductivity of polyethylene: the effects of crystal size, density and orientation on the thermal conductivity. Polym Eng Sci 12:204–208
Choy CL, Chen FC, Luk WH (1980) Thermal conductivity of oriented crystalline polymers. J Polym Sci Polym Phys 18:1187–1207
Merk HJ, Prins JA (1954) Thermal Convection in Laminar Boundary Layers III. Appl Sci Res Sect A 4:207–221
Shanthi V, Agarwal P, Sikand A (2014) Application of heat exchangers in bioprocess industry: a review. Int J Pharm Pharm Sci 6:24–28
Morikawa J, Hashimoto T (1997) Study on thermal diffusivity of poly(ethylene terephthalate) and poly(ethylene naphthalate). Polymer 38:5397–5400
Mannella GA, la Carrubba V, Brucato V (2014) Peltier cells as temperature control elements: experimental characterization and modeling. Appl Therm Eng 63:234–245
Isachenko VP, Osipova VA, Sukomel AS (1987) Heat transfer. MIR, Moscow
Churchill S, Chu H (1975) Correlating equations for laminar and turbulent free convection from a vertical plate. Int J Heat Mass Transf 18:1323–1329
Bejan A, Kraus AD (2003) Heat transfer handbook. Wiley, Hoboken
Elghnam RI (2014) Experimental and numerical investigation of heat transfer from a heated horizontal cylinder rotating in still air around its axis. Ain Shams Eng J 5:177–185
Acknowledgments
This research is part of the DIT (Dynamic Irreversible Thermoporation) project supported by PO FESR 2007/2013 fund of European Union.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix
Appendix
1.1 Internal heat transfer coefficient
The theoretical case of pure diffusive heat transfer inside the bottle was considered.
Although this condition is not expected to be realistic for our case studies, its analysis is still very useful for data interpretation. Indeed, it allows one to define a limit heat transfer coefficient: any experimentally found Ug, if higher than the one calculated for pure diffusive heat transfer, would imply natural convection is occurring within the bottle. External heat transfer was considered extremely fast and PET wall was lumped with internal distilled water. This latter approximation is legit as thermal diffusivity of PET and water are very close (αw = 1.43 × 10−7 and αPET = 1.5 × 10−7) [18]. However, if those thermal diffusivities were not similar, by means of dimensional analysis the PET layer could be substituted with a fictitious water layer [19], thus solving a single diffusion equation instead than two distinct differential equations. In the case of pure conduction, the heat equation written in cylindrical coordinates is [13]:
which must be solved with the following boundary conditions: at t = 0, T = T0; at r = 0, ∂T/∂r = 0; at r = r1 ≈ r2, the conductive heat flux must be equal to the external convective heat flux. The characteristic time for this system, defining the shape of the T versus t curve, can be estimated as τ = R2/α [13]. When using the aforementioned values, calculated τ is around 7600 s. According to theory, after a time close to τ, equilibrium is achieved [20].
A simulation with COMSOL Multiphysics package, assuming pure heat conduction, gave nearly the same results (Fig. 9). The external heat transfer coefficient was set to 1000 W/m2 K, to highlight the internal resistance.
In this way, the maximum cooling time was estimated, related to the worst heat transfer conditions inside the bottle. A lumped system with the same τ, would be characterized by a Ug in the order of 10−2 W/m2 K, therefore any bigger Ug experimentally measured, would reasonably imply the presence of convection phenomena inside the bottle. Typical values for heat transfer coefficient of liquids in natural convection conditions are in the range 10–100 W/m2 K [13]. Collected data always led to Ug in this order of magnitude, thus confirming the presence of natural convection phenomena inside the bottle.
1.2 External heat transfer coefficient
To get an estimate of external heat transfer coefficients during static cooling in air and water, different empirical correlations were employed. Nusselt number (\({\text{Nu}} = \frac{\text{hD}}{\text{k}}\), where D is the cylinder diameter) around horizontal cylinders can be calculated by means of empirical correlations Eq. 10 and 11 [21, 22].
In Eq. 11, C is a constant depending on Prandtl number value (\(\Pr = \frac{{\mu {\text{c}}_{\text{p}} }}{\text{k}}\), where μ is the fluid viscosity), which is 0.436 for air and 0.52 for water at ambient conditions. Rayleigh number is defined as Ra = Gr*Pr, with Gr being the Grashof number (\(Gr = \frac{{g\beta \left( {T_{wall} - T_{\infty } } \right)D^{3} }}{{\nu^{2} }}\), where g is the gravitational acceleration, β the volumetric thermal expansion coefficient, ν the kinematic viscosity, Twall the temperature at bottle surface and T∞ the bulk temperature of cooling fluid).
In order to estimate Nu value in the case of vertically positioned bottle, Eq. 12 was used, which is suitable for vertical surfaces [21] and Eq. 13, for vertical cylinders [22].
Results are summarized in Table 1, Theory and Methodology. Calculations for water led to h2 two orders of magnitude higher than in the case of air.
Calculations worked out in order to estimate heat transfer coefficient in air dynamic cooling were based on the work by [23] on heat transfer from a heated rotating cylinder in still air. During rotation at 200 rpm, Reynolds number around bottle is about 2500, while Gr number is nearly 100,000. In this condition, the system cannot be considered as ruled by forced convection neither by natural convection only, and both Re and Gr numbers are needed in order to estimate Nusselt number. For the considered case, Nu is about 40, thus resulting in a heat transfer coefficient h2 of about 17 W/m2 K.
1.3 Heat transfer across PET layer
The scenario herein presented highlights how much PET layer thickness affects global heat transfer coefficient. Writing Eq. 3 as:
where θ is (T−Tc)/(T0−Tc), the time required to reach a certain dimensionless temperature θ can be calculated as:
By substituting Eq. 1, Eq. 6 was derived:
it can be noticed how cooling time is dependent on PET thickness δ. At high fluid dynamics regimes, where 1/h1 and 1/h2 are much smaller than δ/k, this effect becomes more important, and t is significantly influenced by δ [18].
Rights and permissions
About this article
Cite this article
Liga, A., Montesanto, S., Mannella, G.A. et al. Study on heat transfer coefficients during cooling of PET bottles for food beverages. Heat Mass Transfer 52, 1479–1488 (2016). https://doi.org/10.1007/s00231-015-1652-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-015-1652-x