Abstract
Purpose
SPT-07A is an intravenous injection of (+)-2-borneol being developed for the treatment of acute ischemic stroke. This study aimed to investigate the pharmacokinetics, pharmacodynamics, safety, tolerability, and mass balance of SPT-07A after sequentially administered single and multiple infusions of SPT-07A at 10 mg, 20 mg, or 40 mg.
Methods
This phase I, double-blind, randomized, placebo-controlled, dose-escalation study was conducted in 36 Chinese healthy volunteers. Each cohort enrolled 12 eligible subjects, who were 9:3 randomized to receive SPT-07A or matching placebo during the two study occasions, that is, an initial single-dose occasion followed by a 7-day multiple-dose occasion with a dosing interval of 12 h. Pharmacokinetic, pharmacodynamic assessments regarding effects on the central nervous system (CNS) were performed pre-dose and several times post-dose. Safety and tolerability were evaluated throughout the study for each cohort.
Results
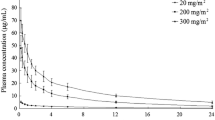
Following single intravenous (i.v.) administration of 10 mg to 40 mg SPT-07A, the plasma SPT-07A concentration reached its peak by the end of infusion. Thereafter, the plasma concentration declined in a multiphase exponential manner with an average terminal elimination half-life of 3.85 to 8.93 h. The exposure parameters of SPT-07A increased dose proportionally. Steady state of SPT-07A was reached after 12-hourly i.v. administrations for 4 days with minimal accumulations. No significant difference of change-from-baseline was observed in the pharmacodynamic measurements between each of the three SPT-07A-treated groups and the placebo group. A total of 41 adverse events (AEs) were reported in 77.8% subjects at 10 mg (7/9), 20 mg (7/9), and 40 mg (7/9), respectively. The AE incidence in placebo group was also 77.8% (7/9). All AEs were mild or moderate in severity and self-limited. SPT-07A was mainly excreted in human urine in glucuronic acid conjugate forms. The total urine recovery rate approximated 84.69% of the administered dose.
Conclusions
SPT-07A was safe and well tolerated after single and multiple intravenous administrations of SPT-07A in the range of 10 mg to 40 mg. SPT-07A presented linear pharmacokinetics in human. Based on plasma exposure, the doses of 10–40 mg twice daily resulted in exposure levels comparable with those obtained at doses demonstrating potential efficacy on AIS animal models and were thus recommended as therapeutic exploratory doses in the phase II clinical trial.


Similar content being viewed by others
References
Ouyang F, Wang Y, Huang W, Chen Y, Zhao Y, Dang G, Zhang C, Lin Y, Zeng J (2018) Association between socioeconomic status and post-stroke functional outcome in deprived rural southern China: a population-based study[J]. BMC Neurol 18(1):12
Fisher M, Feuerstein G, Howells DW et al (2009) Update of the stroke therapy academic industry roundtable preclinical recommendations[J]. Stroke. 40:2244–2250
Jauch EC, Saver JL, Adams HP et al (2013) Guidelines for the early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association[J]. Stroke. 44(3):870–947
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators (2008) Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke[J]. N Engl J Med 359(13):1317–1329
Bin Z, Xiao-Jiang S, Chi-Heng J (2011) Thrombolysis with alteplase 4.5–6 hours after acute ischemic stroke[J]. Eur Neurol 65(3):170
Hussein HM, Georgiadis AL, Vazquez G, Miley JT, Memon MZ, Mohammad YM, Christoforidis GA, Tariq N, Qureshi AI (2010) Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study[J]. AJNR Am J Neuroradiol 31(3):454–458
Zoppo GJD, Saver JL, Jauch EC et al (2009) Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association[J]. Stroke 40(8):2945–2948
Dk J et al (1999) Clinical trials with neuroprotective drugs in acute ischemic stroke: are we doing the right thing?[J]. Trends Neurosci 22(12):535–540
Marshall RS (2015) Progress in intravenous thrombolytic therapy for acute stroke[J]. JAMA Neurol 72(8):928–934
Watanabe T, Tahara M, Todo S (2010) The novel antioxidant edaravone: from bench to bedside[J]. Cardiovasc Ther 26(2):101–114
Wang JK, Chen X, Yuan B, Wang W, Xu C, Zhao W, Zhao P, Wang Y, Zhao X, Wang Y (2018) Bioavailability of edaravone sublingual tablet versus intravenous infusion in healthy male volunteers[J]. Clin Ther 40(10):1683–1691
Hiu T, Farzampour Z, Paz JT, Wang EH, Badgely C, Olson A, Micheva KD, Wang G, Lemmens R, Tran KV, Nishiyama Y, Liang X, Hamilton SA, O'Rourke N, Smith SJ, Huguenard JR, Bliss TM, Steinberg GK (2016) Enhanced phasic GABA inhibition during the repair phase of stroke: a novel therapeutic target[J]. Brain. 139(Pt 2):468–480
Clarkson AN, Huang BS, Macisaac SE et al (2010) Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke[J]. Nature. 468(7321):305–309
Liu J, Wang LN, Ma X, Ji X (2016) Gamma aminobutyric acid (GABA) receptor agonists for acute stroke[J]. Cochrane Database Syst Rev 10:CD009622
Granger RE, Campbell EL et al (2005) (+) And (−) borneol: efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors[J]. Biochem Pharmacol 69:1101–1111
Macleod MR, Fisher M, O’Collins V et al (2009) Good laboratory practice: preventing introduction of bias at the bench[J]. Stroke. 40:e50–e52
Du QQ, Wang ZJ, He L et al (2013) PXR polymorphisms and their impact on pharmacokinetics/pharmacodynamics of repaglinide in healthy Chinese volunteers[J]. Eur J Clin Pharmacol 69(11):1917–1925
Lee SK, Xing J, Catlett IM, Adamczyk R, Griffies A, Liu A, Murthy B, Nowak M (2017) Safety, pharmacokinetics, and pharmacodynamics of BMS-986142, a novel reversible BTK inhibitor, in healthy participants[J]. Eur J Clin Pharmacol 73(6):689–698
Chen X, Jacobs G, de Kam M, Jaeger J, Lappalainen J, Maruff P, Smith MA, Cross AJ, Cohen A, van Gerven J (2014) The central nervous system effects of the partial GABA-Aα2,3 -selective receptor modulator AZD7325 in comparison with lorazepam in healthy males[J]. Br J Clin Pharmacol 78(6):1298–1314
Chen X, van Gerven J, Cohen A, Jacobs G (2019) Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety[J]. Acta Pharmacol Sin 40(5):571–582
Zuiker RG, Chen X, Østerberg O, Mirza NR, Muglia P, de Kam M, Klaassen ES, van Gerven J (2016) NS11821, a partial subtype-selective GABAA agonist, elicits selective effects on the central nervous system in randomized controlled trial with healthy subjects[J]. J Psychopharmacol 30(3):253–262
Chen X, de Haas S, de Kam M, van Gerven J (2012) An overview of the CNS-pharmacodynamic profiles of non-selective and selective GABA receptor modulators[J]. Adv Pharmacol Sci 2012:134523. https://doi.org/10.1155/2012/134523
Chen X, Sang N, Song KC et al Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote[J]. Clin Ther Accepted
Chen X, Jacobs G, de Kam ML et al (2015) AZD6280, a novel partial γ-aminobutyric acid A receptor modulator, demonstrates a pharmacodynamically selective effect profile in healthy male volunteers[J]. J Clin Psychopharmacol 35(1):22–33
Chen X, Broeyer F, de Kam M, Baas J, Cohen A, van Gerven J (2017) Pharmacodynamic response profiles of anxiolytic and sedative drugs[J]. Br J Clin Pharmacol 83(5):1028–1038
de Haas SL, Franson KL, Schmitt JA, Cohen AF, Fau JB, Dubruc C, van Gerven JM (2009) The pharmacokinetic and pharmacodynamic effects of SL65.1498, a GABAA 2,3 selective receptor modulator, in comparison with lorazepam in healthy volunteers[J]. J Psychopharmacol 23:625–632
Tanious MK, Beutler SS, Kaye AD, Urman RD (2017) New hypnotic drug development and pharmacologic considerations for clinical anesthesia[J]. Anesthesiol Clin 35(2):e95
Administration FD (2005) Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers[J].
Acknowledgments
The authors would like to thank Suzhou Pharmavan Co., Ltd. for sponsoring this study. We also would like to express our gratitude to all the volunteers who participated in the study for your contribution to medicine and to science.
Funding
This study was supported by National Grant for New Drug Development 2017ZX09304018 and Chinese National Natural Fund Grant 81671369.
Author information
Authors and Affiliations
Contributions
Xia Chen and Yongjun Wang designed the research. Xia Chen and Weicong Wang wrote the clinical trial protocol. Xia Chen, Weicong Wang, Yan Wang, Weiwei Zhao, and Jingbo Zhong performed the research. Weicong Wang wrote the manuscript. All authors contributed towards critically revising the manuscript for important intellectual content, approve the final manuscript, and agree to be accountable for all aspects of the work and will ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author statement
The authors confirm that the Principal Investigator for this paper is Xia Chen and Yongjun Wang and that they had direct clinical responsibility for patients.
Rights and permissions
About this article
Cite this article
Wang, W., Wang, Y., Zhao, W. et al. Pharmacokinetics, pharmacodynamics, safety, tolerability, and mass balance of single and continuous intravenous infusion of SPT-07A in healthy volunteers. Eur J Clin Pharmacol 76, 785–793 (2020). https://doi.org/10.1007/s00228-020-02851-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02851-x