Abstract
Purpose
Ilaprazole, the latest proton pump inhibitor, can be used with clarithromycin and amoxicillin as a triple therapy regimen for eradicating Helicobacter pylori. The aim of this study was to evaluate pharmacokinetic drug interactions and safety profiles after coadministration of clarithromycin, amoxicillin, and ilaprazole.
Methods
A randomised, open-label, one-way crossover, two parallel sequences study was conducted in 32 healthy subjects. In part 1, the subjects received a single dose of ilaprazole 10 mg in period 1 and clarithromycin 500 mg and amoxicillin 1000 mg twice daily for 6 days in period 2. In part 2, the subjects received clarithromycin 500 mg and amoxicillin 1000 mg once in period 1 and ilaprazole 10 mg twice daily for 6 days in period 2. In both sequences, the three drugs were coadministrated once on day 5 in period 2. Pharmacokinetic evaluations of ilaprazole (part 1), and clarithromycin and amoxicillin (part 2) were conducted.
Results
Twenty-eight subjects completed the study. For ilaprazole, the peak concentration (Cmax) slightly decreased from 479 (ilaprazole alone) to 446 ng/mL (triple therapy) [Geometric least square mean ratio (90% confidence interval), 0.93 (0.70–1.22)]. The area under the concentration-time curve from 0 h to the last measurable concentration (AUClast) slightly increased from 3301 to 3538 μg·h/mL [1.07 (0.85–1.35)]. For clarithromycin, the Cmax slightly decreased from 1.87 to 1.72 μg/mL [0.90 (0.70–1.15)], and AUClast slightly increased from 14.6 to 16.5 μg·h/mL [1.09 (0.87–1.37)]. For amoxicillin, the Cmax slightly decreased from 9.37 to 8.14 μg/mL [0.86 (0.74–1.01)], and AUClast slightly decreased from 27.9 to 26.7 μg·h/mL [0.98 (0.83–1.16)]. These changes in the PK parameters of each drug were not statistically significant.
Conclusions
The coadministration of ilaprazole, clarithromycin, and amoxicillin was tolerable and did not cause a significant PK drug interaction. Thus, a triple therapy regimen comprising ilaprazole, clarithromycin, and amoxicillin may be an option for the eradication of H. pylori.
Clinicaltrials.gov number: NCT02998437.
Similar content being viewed by others
Introduction
Peptic ulcer disease (PUD), the most common disease related to gastric acid, develops due to an imbalance of aggressive gastric luminal factors (e.g. acid and pepsin) and defensive mucosal barrier function. PUD includes both gastric and duodenal ulcers characterised by epigastric pain, fullness, bloating, early satiety, and nausea [1]. Helicobacter pylori infection is found in 60–90% of patients with peptic ulcer [2]. A strong association between H. pylori infection and PUD has been shown in epidemiological studies [3, 4]. The eradication of H. pylori was found essential for the permanent cure for peptic ulcers [3, 4]. Thus, the eradication of H. pylori has become an important factor in PUD treatment.
In addition to PUD, H. pylori is associated with many gastrointestinal diseases, such as chronic gastritis, gastric adenocarcinoma, and lymphoma. With the recognition of the clinical importance of H. pylori, numerous therapeutic regimens have been evaluated for eradication of H. pylori. Although eradication failure due to antibiotic resistance has been a concern recently, clinical guidelines recommend a clarithromycin-containing standard triple regimen as the first-line treatment for H. pylori [1, 2]. The standard triple therapy consists of proton pump inhibitors (PPIs), clarithromycin, and amoxicillin [5], which has achieved H. pylori eradication rates of 71–80% [6, 7]. However, varying degrees of drug interactions have been observed between coadministered PPIs and antibiotics.
Clarithromycin is a potent cytochrome P450 (CYP) 3A4 inhibitor, and affects most PPIs (such as omeprazole, lansoprazole, and esomeprazole) that are metabolised by CYP3A4 [8,9,10]. PPIs may, in turn, alter the metabolism of concomitantly administered antibiotics via either CYP enzyme inhibition or a change in the pH-dependent solubility of the drugs [11]. It has been demonstrated that omeprazole attenuates the breakdown of acid-labile antibacterials such as amoxicillin and increases their intragastric concentrations [12]. Based on this finding, it has been speculated that PPIs may increase the bioavailability of acid-labile antibiotics.
Ilaprazole, the latest PPI developed by IL-YANG Pharmaceutical Co., Ltd. (Seoul, South Korea) and approved for the treatment of duodenal ulcers, gastric ulcers, and erosive esophagitis, has the longest half-life among PPIs [13]. Furthermore, it shows greater and prolonged suppression of gastric acid secretion even at a dose of 10 mg that is higher than that achieved with a 20-mg dose of omeprazole [14, 15]. Considering that a double dose of PPIs is superior to a single dose when used as part of triple therapy for H. pylori eradication [16], triple therapy including ilaprazole, a more potent PPI, is expected to be effective in H. pylori eradication.
In the early period of drug development, in vitro studies reported that ilaprazole was reported from in vitro studies to be mainly metabolised by CYP3A4 among the CYP isoforms, and converted to the major metabolite, ilaprazole sulfone [17,18,19]. CYP2C19 has been revealed to be rarely involved in ilaprazole metabolism unlike that observed for other PPIs such as omeprazole, esomeprazole, pantoprazole, and lansoprazole [13, 19]. However, a new metabolite, ilaprazole thiol ether, formed via a non-enzymatic metabolic pathway was identified in human studies after ilaprazole administration, and even ilaprazole thiol ether was detected in large or similar amounts compared to those of ilaprazole sulfone [20, 21]. These results indicate that ilaprazole has various metabolic pathways consisting of both CYP-mediated enzymatic and non-enzymatic metabolic pathways, and the non-enzymatic pathway plays a significant role in ilaprazole metabolism. The differences in the metabolic pathway between ilaprazole and other PPIs could also cause differences in drug interactions with coadministered antibiotics for H. pylori eradication.
There has been a great demand from clinicians to use ilaprazole in H. pylori eradication therapy, but the drug interactions between ilaprazole and coadministered antibiotics in the standard triple therapy (e.g. clarithromycin and amoxicillin) have not yet been clearly determined. In this study we evaluated the pharmacokinetic (PK) drug interaction between clarithromycin, amoxicillin, and ilaprazole intended for a triple therapy regimen for eradication of H. pylori.
Subjects and methods
Study subjects
Healthy Korean male volunteers who were aged between 19 and 50 years with a body mass index between 18.5 and 25.0 kg/m2 were enrolled in the study. Volunteers went through the following screening procedures: taking the medical history, physical examination, 12-lead electrocardiography (ECG), and assessment of clinical laboratory parameters including haematology, blood chemistry, and urine drug screening after they voluntarily signed the informed consent form. The participants were not allowed to consume alcohol, caffeinated beverage, and grapefruit products or smoke during the study period. Any ethical drugs or herbal medicines were prohibited for 2 weeks before the study drug administration.
Study design, treatments, and administration
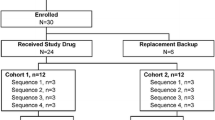
This was a randomised, open-label, one-way crossover, two parallel sequences study. A total of 32 subjects were randomised and assigned to one of the two parts. Subjects assigned to part 1 received a single dose of ilaprazole (Noltec Tab., IL-YANG Pharmaceutical Co.) 10 mg in the first period. Then, after a washout period of 10 days, clarithromycin (Klaricid Film Coated Tab., Abbott Korea) 500 mg and amoxicillin (Amoxicillin Cap. 500 mg Chongkundang, Chong Kun Dang Pharmaceutical Co., Korea) 1000 mg were administered twice daily for 6 days in the second period. Ilaprazole 10 mg was coadministered with clarithromycin and amoxicillin on the day 5 of the second period.
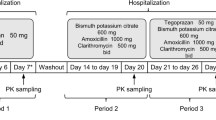
Subjects assigned to part 2 received single doses of both clarithromycin 500 mg and amoxicillin 1000 mg in the first period; after a washout period of 10 days, ilaprazole 10 mg was administered twice daily for 6 days in the second period. Clarithromycin 500 mg and amoxicillin 1000 mg were coadministered with ilaprazole on the day 5 of the second period (Fig. 1). All study drugs were administered via the oral route, and the dosage of each drug was determined based on the results of a previous ilaprazole phase 3 study, treatment guidelines for H. pylori eradication, and labels of clarithromycin and amoxicillin [22,23,24,25,26,27].
Study design. Notes: Subjects who were assigned to part 1 received ilaprazole 10 mg on day 1 of period 1 and clarithromycin 500 mg and amoxicillin 1000 mg from day 1 to day 6 of period 2 and ilaprazole 10 mg was concomitantly administered on day 5 of period 2. Subjects who were assigned to part 2 received both clarithromycin 500 mg and amoxicillin 1000 mg on day 1 of period 1 and ilaprazole 10 mg from day 1 to day 6 of period 2 and clarithromycin 500 mg and amoxicillin 1000 mg was concomitantly administered on day 5 of period 2
The study was approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine, Seoul, Korea, and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
Blood sample collection and analysis
Serial blood samples were collected in anti-coagulated EDTA-K2 tubes at 0 (pre-dose), 1, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, 12, 24, and 48 h after dosing of ilaprazole in part 1. The PK sampling points in part 2 were 0 (pre-dose), 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, and 48 h after dosing of clarithromycin and amoxicillin. Blood sampling points were determined considering the time to reach peak concentration (Tmax) and elimination half-life (t1/2) of ilaprazole, clarithromycin, and amoxicillin [9, 10, 20]. Plasma concentrations of ilaprazole, clarithromycin, and amoxicillin were analysed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Samples were centrifuged for 10 min under 4 °C at 2000×g and stored below − 70 °C until analysis.
For the analysis of ilaprazole, 100 μL of plasma was added to polypropylene tubes and mixed with 10 μL of the internal standard (omeprazole) solution and 1 mL of acetonitrile. The mixture was vortexed for 1 min and centrifuged for 5 min at 11,337×g. The supernatant (100 μL) was collected and reconstituted with 600 μL of mobile phase. The supernatant (2 μL) was then injected into an LC-MS/MS system. An LC system (Shiseido Nanospace SI-2, Shiseido, Tokyo, Japan) was used with a mobile phase (10 mM ammonium formate/acetonitrile = 50/50 [v/v]) at 40 °C. Chromatographic separation was performed at a flow rate of 0.25 mL/min. The transition of ilaprazole was detected at m/z 367.0 to 184.2 by using an MS/MS system (API4000®, AB SCIEX, Washington DC, USA) in the multiple reaction monitoring (MRM) mode with a positive electrospray ionisation (ESI) source. The calibration curve was linear over the range of 5–1000 ng/mL, and the correlation coefficient (r) was > 0.995.
For the analysis of clarithromycin, 100 μL of plasma was added to polypropylene tubes and mixed with 10 μL of the internal standard (clarithromycin-N-methyl-13C, d3) solution and 500 μL of acetonitrile. The mixture was vortexed for 1 min and centrifuged for 5 min at 11,337×g. The supernatant (50 μL) was collected and reconstituted with 1000 μL of 50% acetonitrile. The supernatant (3 μL) was then injected into the LC-MS/MS system. The LC system was used with a mobile phase (10 mM ammonium formate/acetonitrile = 20/80 [v/v]) at 40 °C. Chromatographic separation was performed at a flow rate of 0.2 mL/min. The transition of clarithromycin was detected at m/z 748.4 to 590.4 using an MS/MS system (4000QTRAP, AB SCIEX, Washington DC, USA) in the MRM mode with a positive ESI source. The calibration curve was linear over the range of 10–7000 ng/mL, and the correlation coefficient (r) was > 0.995.
For the analysis of amoxicillin, 100 μL of plasma was added to polypropylene tubes and mixed with 10 μL of internal standard (amoxicillin-d4) solution and 500 μL of acetonitrile. The mixture was vortexed for 1 min and centrifuged for 5 min at 11,337×g. The supernatant (100 μL) was collected and reconstituted with 100 μL of 50% acetonitrile. The supernatant (5 μL) was injected into the LC-MS/MS system. The LC system was used with a mobile phase (deionised water/acetonitrile/formic acid = 70:30:0.1 [v/v/v]) at 40 °C. Chromatographic separation was performed at a flow rate of 0.2 mL/min. The transition of amoxicillin was detected at m/z 366.2 to 208.2 using an MS/MS system (4000QTRAP, AB SCIEX, Washington DC, USA) in the MRM mode with a positive ESI source. The calibration curve was linear over the range of 0.1–50 μg/mL, and the correlation coefficient (r) was > 0.995.
The coefficient of variation (CV) represents the overall precision of the assay of the three study drugs, which was < 20.0% for the lower limit of quantification (LLOQ) and 15.0% for other upper concentrations for calibration.
PK assessment
PK parameters were evaluated using a non-compartmental model of Phoenix WinNonlin® software (version 6.4; Certara, St. Louis, MO, USA). The peak concentration (Cmax) and Tmax were determined directly from the observed values. The area under the curve from the time of dosing to the last measurable concentration (AUClast) was calculated using the linear trapezoidal rule. The terminal elimination rate constant (λz) was estimated by the linear regression analysis of the terminal portion of the log-transformed plasma concentration-to-time profile. The elimination half-life (t1/2), apparent plasma clearance (CL/F), and apparent volume of distribution (Vd/F) were calculated using the following equations t1/2 = ln(2)/λz, CL/F = dose/AUCinf, and Vd/F = dose/(λz · AUCinf), respectively. The AUC of the last dosing time extrapolated to infinity (AUCinf) was calculated using the formula AUClast + Clast/λz, where Clast represents the last measurable concentration. The PK drug interactions after triple therapy were evaluated by comparing the plasma concentration-time profiles and PK parameters of each drug.
Safety assessment
Safety and tolerability were evaluated throughout the study. The assessments included monitoring of all adverse events (AEs) based on physical examinations, vital signs, 12-lead ECG, routine haematology, serum chemistry, and urinalysis. The subjects were instructed to notify the study physicians or nurses of any AEs that occurred during the study. All AEs reported by subjects or detected in the assessments were recorded, and the investigators determined their relationship to the treatment. The severity of the AEs was evaluated based on the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03).
Statistical analysis
Because this study was explorative and sought to evaluate the PK interactions of the study drugs, it was deemed preferable to use minimum number of subjects. Considering that the power of the parallel study design would be insufficient compared with that of the crossover design, the minimum number of subjects was estimated to be 28, consisting of 14 subjects/per part, and the total number of subjects was 32 (16 subjects/part) under the assumption of a potential drop-out rate of 15%. Descriptive statistics were calculated for baseline demographics, PK parameters, and safety data by part. Baseline demographics of the treatment sequences were evaluated using the Student’s t test using the SAS statistical software version 9.4 (SAS Institute Inc. Cary, NC, USA). To compare the PK parameters (Cmax, AUClast, and AUCinf) between treatments, a general linear mixed effects model was constructed using log-transformed data with treatment as the fixed effect and variance within subjects as a random effect. The geometric least squares mean ratio (GMR) and the 90% confidence interval (CI) of the Cmax, AUClast, and AUCinf between the two different treatments of each part were determined. All statistical analyses were conducted using SAS® version 9.4. All statistical tests were two-sided, and a P < 0.05 was considered statistically significant.
Results
Baseline demographics
A total of 34 subjects were enrolled in this study. Two subjects were dropped before the investigational products were administered because of clinical laboratory test results, and 32 subjects received the investigational products. Each part consisted of 16 subjects, and two subjects were dropped during period 1 because they matched an exclusion criterion (smoking during the PK assessment period) and one was dropped after period 1 owing to herpes zoster in part 1 (in all, three subjects were dropped). One subject was dropped during period 1 because of smoking in part 2. Thus, 28 subjects completed the study and were included in the PK analysis. In contrast, 32 subjects who were administered at least one dose of any investigational product were included in the safety analysis. The demographic information of the study subjects is presented in Supplementary Table 1.
Pharmacokinetics
The mean plasma concentration-time profiles of ilaprazole, clarithromycin, and amoxicillin are shown in Figs. 2, 3, and 4. The PK parameters of each study drug and the point estimate with the 90% CI of the GMR of triple therapy to monotherapy or dual therapy are summarised in Table 1.
Mean (SD) plasma concentration-time profiles of ilaprazole when administered as monotherapy and as part of triple therapy. a Linear scale; b semi-logarithmic scale. Abbreviations: monotherapy, administration of ilaprazole 10 mg in period 1 of treatment part 1; triple therapy, administration of ilaprazole 10 mg, clarithromycin 500 mg, and amoxicillin 1000 mg in period 2 of both treatment parts 1 and 2
Mean (SD) plasma concentration-time profiles of clarithromycin when administered as dual therapy and as part of triple therapy. a Linear scale; b semi-logarithmic scale. Abbreviations: dual therapy, administration of clarithromycin 500 mg and amoxicillin 1000 mg in period 1 of treatment part 2; triple therapy, administration of ilaprazole 10 mg, clarithromycin 500 mg, and amoxicillin 1000 mg in period 2 of both treatment parts 1 and 2
Mean (SD) plasma concentration-time profiles of amoxicillin when administered as dual therapy and as part of triple therapy. a Linear scale; b semi-logarithmic scale. Abbreviations: dual therapy, administration of clarithromycin 500 mg and amoxicillin 1000 mg in period 1 of treatment part 2; triple therapy, administration of ilaprazole 10 mg, clarithromycin 500 mg, and amoxicillin 1000 mg in period 2 of both treatment parts 1 and 2. Notes: Plasma amoxicillin concentrations at 48 h after study drug administration were not detected
The mean Cmax values of ilaprazole administered as part of the triple therapy compared to the monotherapy were 446 and 479 ng/mL, and the mean Tmax values were 3.0 and 3.5 h after dosing, respectively. The corresponding mean AUClast values was 3538 and 3301 ng·h/mL, and the mean AUCinf values were 3681 and 3393 ng·h/mL, respectively. Although the Cmax of ilaprazole slightly decreased and the AUClast slightly increased with the triple therapy, those changes were not statistically significant.
The mean Cmax of clarithromycin was 1.72 and 1.87 μg/mL with the triple and dual therapies, respectively. The mean Tmax, AUClast, and AUCinf values of the triple and dual therapies were 2.5 and 2.0 h after dosing, 16.5 and 14.6 μg·h/mL, and 16.9 and 15.0 μg·h/mL, respectively. Similar to the changes in PK data of ilaprazole, the Cmax of clarithromycin slightly decreased, and the AUClast slightly increased; however, the changes were not statistically significant.
For amoxicillin, the mean Cmax values of amoxicillin were 8.14 and 9.37 μg/mL with the triple and dual therapies. Furthermore, the corresponding mean Tmax, AUClast, AUCinf values for the triple and dual therapy were 2.0 and 1.5 h after dosing, 26.7 and 27.9 μg·h/mL, and 27.4 and 28.5 μg·h/mL, respectively. The Cmax and AUClast of amoxicillin slightly decreased with the triple therapy. However, the changes were also not statistically significant.
All three drugs were determined not to be significantly affected by the triple coadministration, because the changes in PK data of each drug were clinically and statistically insignificant.
Safety assessment
A total of 35 AEs were reported by 20 subjects (62.5%), and they are summarised in Table 2. Nine of them (28.1%) were considered to be related to the study drugs, and there was no drug-related AE when subjects received ilaprazole alone. The incidence of drug-related AEs increased with the administration of the triple therapy in each part, but it was not clinically significant since the symptoms were mild and transient. There were no serious AEs, and all subjects recovered without complications. There were no clinically significant differences in vital signs, physical examinations, laboratory tests, and other observations related to safety in both parts. The AEs reported after the administration of the investigational products were generally consistent with the known safety profiles of ilaprazole, clarithromycin, and amoxicillin.
Discussion
This study showed that there were no significant PK drug interactions between clarithromycin or amoxicillin and ilaprazole. Based on the results from in vitro studies that ilaprazole is mainly metabolised by CYP3A4 among CYP-isofoms, our result demonstrating that clarithromycin showed no significant drug interaction with ilaprazole poses a question. However, it does not negate that clarithromycin influences the metabolism of ilaprazole. In our result, the exposure and elimination of ilaprazole were slightly affected by the triple therapy. The AUClast slightly increased, the half-life was slightly prolonged, and the clearance (CL/F) was slightly reduced, although those findings were not of a statistical significance. It suggests that the metabolism of ilaprazole might be influenced by clarithromycin, but within a small extent. The influence of clarithromycin on the exposure of ilaprazole could be limited if another metabolic pathway (e.g. non-enzymatic pathway) plays a considerable part in the metabolism of ilaprazole. Although we did not measure the concentration of metabolites in this study, previous studies revealed that ilaprazole sulfone and thiol ether were detected in comparable amounts when ilaprazole was administered to human [20, 21]. This implies that non-enzymatic pathway might not be minor. Taken together, these results led us to speculate that clarithromycin might affect the metabolism of ilaprazole, but its influence was limited, because ilaprazole could be metabolised by non-enzymatic pathway.
Regarding drug interaction in the absorption of ilaprazole, a previous study by Cao et al. reported that the Cmax and the AUC of ilaprazole and ilaprazole sulfone significantly decreased following the triple therapy, while ilaprazole thiol ether, another metabolite of ilaprazole, did not change [20]. Cao et al. speculated that the decrease in absorption of ilaprazole was the mechanism of their result, because the half-life and the clearance of ilaprazole were not affected. However, we could not identify a significant decrease in the absorption of ilaprazole in our study, although its Cmax slightly decreased. We suggest that follow-up studies are needed to validate the influence of clarithromycin or amoxicillin on the absorption of ilaprazole.
Proton pump inhibitors may also have an inhibitory effect on CYP3A4 activity and increase the exposure of drugs metabolised mainly by CYP3A4 [11]. However, this effect varies among PPIs. It has been revealed that omeprazole and lansoprazole increase the exposure of clarithromycin when coadministered [8, 9]. Pantoprazole was shown to be a competitive inhibitor of CYP3A4 via in vitro study, but the metabolic pathway has not yet been demonstrated in human studies [11]. Rabeprazole did not show any inhibitory effect on CYP isoforms, but the non-enzymatically formed metabolite of rabeprazole, rabeprazole thioether, was revealed to have an inhibitory effect on CYP2C9, CYP2C19, CYP2D6, and CYP3A4 [28]. However, the plasma concentration of clarithromycin was largely unchanged by combination therapy with esomeprazole [10]. As in the case of esomeprazole, the exposure of clarithromycin was not significantly affected by ilaprazole in our study.
In addition to the anti-acid secretory effect, PPIs are considered to enhance the anti-H. pylori activities of concomitantly administered amoxicillin and clarithromycin in H. pylori eradication therapy. It has been demonstrated that PPIs enhance the stability of acid-labile antibiotics (e.g. penicillins) by increasing the gastric pH [12]. Based on this finding, it has been speculated that PPIs may increase the bioavailability of amoxicillin in triple therapy [11]. Despite this prediction, there are no reports that demonstrate any significant changes in the exposure of amoxicillin in the drug interaction studies between amoxicillin and PPIs [9, 10, 20, 29, 30]. Our result also showed no clinically significant changes in the exposure of amoxicillin except a slight decrease in the Cmax (GMR, 0.86; 90% CI, 0.74–1.01). It would be difficult to demonstrate further increase in amoxicillin exposure by increasing pH, considering already high bioavailability of amoxicillin (89–98%) [31] and the single dosing of amoxicillin in our study.
Although someone might speculate that the PK interactions between PPIs and clarithromycin resulting in increased exposures of both drugs may contribute to higher eradication rates [20], drug interactions causing excessive exposure may be detrimental to defining the appropriate dosage regimen and may result in undesirable increases in adverse events. In this respect, our results support that ilaprazole, the exposure of which is only minimally influenced by coadministration of clarithromycin and amoxicillin, may have advantages over other PPIs to be used in triple therapy regimen.
Taken together, the results of our study indicate that there were no clinically significant PK drug interactions between clarithromycin, amoxicillin, and ilaprazole. Thus, the standard triple therapy with ilaprazole could be a desirable treatment option for H. pylori eradication. The limitation of our study is that the subjects were limited to healthy male Korean population. Because there are relevant ethnicity-related differences in PPI metabolism [32], further studies are required to evaluate whether there is an ethnicity- or sex-related difference in the drug interactions of ilaprazole.
Conclusions
The coadministration of ilaprazole, clarithromycin, and amoxicillin was tolerable and did not cause a significant PK drug interaction. Thus, a triple therapy regimen comprising ilaprazole, clarithromycin, and amoxicillin may be an option for the eradication of H. pylori.
References
Malfertheiner P, Chan FK, McColl KE (2009) Peptic ulcer disease. Lancet 374:1449–1461
Lee DH (2002) Current status and treatment of Helicobacter pylori infection in Korea. Korean J Gastroenterol 39:153–160
Rauws EA, Tytgat GN (1990) Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet 335:1233–1235
Malfertheiner P, Leodolter A, Peitz U (2000) Cure of Helicobacter pylori-associated ulcer disease through eradication. Baillieres Best Pract Res Clin Gastroenterol 14:119–132
Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS (2009) Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol 54:269–278
Sasaki M, Ogasawara N, Utsumi K, Kawamura N, Kamiya T, Kataoka H, Tanida S, Mizoshita T, Kasugai K, Joh T (2010) Changes in 12-year first-line eradication rate of Helicobacter pylori based on triple therapy with proton pump inhibitor, amoxicillin and clarithromycin. J Clin Biochem Nutr 47:53–58
Jung YS, Park CH, Park JH, Nam E, Lee HL (2017) Efficacy of Helicobacter pylori eradication therapies in Korea: a systematic review and network meta-analysis. Helicobacter 22
Calabresi L, Pazzucconi F, Ferrara S, Di Paolo A, Tacca MD, Sirtori C (2004) Pharmacokinetic interactions between omeprazole/pantoprazole and clarithromycin in health volunteers. Pharmacol Res 49:493–499
Mainz D, Borner K, Koeppe P, Kotwas J, Lode H (2002) Pharmacokinetics of lansoprazole, amoxicillin and clarithromycin after simultaneous and single administration. J Antimicrob Chemother 50:699–706
Hassan-Alin M, Andersson T, Niazi M, Liljeblad M, Persson BA, Rohss K (2006) Studies on drug interactions between esomeprazole, amoxicillin and clarithromycin in healthy subjects. Int J Clin Pharmacol Ther 44:119–127
Ogawa R, Echizen H (2010) Drug-drug interaction profiles of proton pump inhibitors. Clin Pharmacokinet 49:509–533
Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idstrom JP, Cederberg C, Spiller RC (1996) Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology 111:358–367
de Bortoli N, Martinucci I, Giacchino M, Blandizzi C, Marchi S, Savarino V, Savarino E (2013) The pharmacokinetics of ilaprazole for gastro-esophageal reflux treatment. Expert Opin Drug Metab Toxicol 9:1361–1369
Du YQ, Guo WY, Zou DW et al (2012) Acid inhibition effect of ilaprazole on Helicobacter pylori-negative healthy volunteers: an open randomized cross-over study. J Dig Dis 13:113–119
Periclou AP, Goldwater R, Lee SM, Park DW, Kim DY, Cho KD, Boileau F, Jung WT (2000) A comparative pharmacodynamic study of IY-81149 versus omeprazole in patients with gastroesophageal reflux disease. Clin Pharmacol Ther 68:304–311
Vallve M, Vergara M, Gisbert JP, Calvet X (2002) Single vs. double dose of a proton pump inhibitor in triple therapy for Helicobacter pylori eradication: a meta-analysis. Aliment Pharmacol Ther 16:1149–1156
Li Y, Zhang W, Guo D, Zhou G, Zhou H, Xiao Z (2008) Pharmacokinetics of the new proton pump inhibitor ilaprazole in Chinese healthy subjects in relation to CYP3A5 and CYP2C19 genotypes. Clin Chim Acta 391:60–67
Myung SW, Min HK, Jin C, Kim M, Lee SM, Chung GJ, Park SJ, Kim DY, Cho HW (1999) Identification of IY81149 and its metabolites in the rat plasma using the on-line HPLC/ESI mass spectrometry. Arch Pharm Res 22:189–193
Seo KA, Lee SJ, Kim KB, Bae SK, Liu KH, Kim DH, Shin JG (2012) Ilaprazole, a new proton pump inhibitor, is primarily metabolized to ilaprazole sulfone by CYP3A4 and 3A5. Xenobiotica 42:278–284
Cao S, Zhou G, Ou-Yang DS et al (2012) Pharmacokinetic interactions between ilaprazole and clarithromycin following ilaprazole, clarithromycin and amoxicillin triple therapy. Acta Pharmacol Sin 33:1095–1100
Zhou G, Tan ZR, Zhang W, Ou-Yang DS, Chen Y, Guo D, Liu YZ, Fan L, Deng HW (2009) An improved LC-MS/MS method for quantitative determination of ilaprazole and its metabolites in human plasma and its application to a pharmacokinetic study. Acta Pharmacol Sin 30:1330–1336
Ho KY, Kuan A, Zano F et al (2009) Randomized, parallel, double-blind comparison of the ulcer-healing effects of ilaprazole and omeprazole in the treatment of gastric and duodenal ulcers. J Gastroenterol 44:697–707
Song J, Guo B, Yao L, Tang J (2010) S1046 the clinical study of Ilaprazole on duodenal ulcer, a randomize study compared with esomeprazole. Gastroenterology 138:S-166–S-167
U.S. Food and Drug Administration. Amoxil capsules, Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050542s24,050754s11,050760s10,050761s10lbl.pdf. Accessed Nov 24 2017
U.S. Food and Drug Administration. Biaxin tablet, Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050662s042,050698s024,050775s013lbl.pdf
Wang L, Zhou L, Hu H, Lin S, Xia J (2012) Ilaprazole for the treatment of duodenal ulcer: a randomized, double-blind and controlled phase III trial. Curr Med Res Opin 28:101–109
Xue Y, Qin X, Zhou L, Lin S, Wang L, Hu H, Xia J (2016) A randomized, double-blind, active-controlled, multi-center study of Ilaprazole in the treatment of reflux esophagitis. Clin Drug Investig 36:985–992
Li XQ, Andersson TB, Ahlstrom M, Weidolf L (2004) Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 32:821–827
Furuta T, Shirai N, Takashima M, Xiao F, Hanai H, Sugimura H, Ohashi K, Ishizaki T, Kaneko E (2001) Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther 69:158–168
Gustavson LE, Kaiser JF, Edmonds AL, Locke CS, DeBartolo ML, Schneck DW (1995) Effect of omeprazole on concentrations of clarithromycin in plasma and gastric tissue at steady state. Antimicrob Agents Chemother 39:2078–2083
Sanchez Navarro A (2005) New formulations of amoxicillin/clavulanic acid: a pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet 44:1097–1115
Chong E, Ensom MH (2003) Pharmacogenetics of the proton pump inhibitors: a systematic review. Pharmacotherapy 23:460–471
Acknowledgements
We thank the staff of the Clinical Trials Center of Severance Hospital for their generous cooperation.
Funding
This study was sponsored by IL-YANG Pharmaceutical Co., Ltd., Yong-in, Korea, and supported by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1062).
Author information
Authors and Affiliations
Contributions
BHJ and BWY wrote the manuscript; BHJ, BWY, JHK, JYL, JSS, and MSP designed the study; BHJ, BWY, and MSP performed the research; BHJ, BWY, JSP, and MSP analysed the data. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Jung Hye Kim, Jun Yeon Lee, and Jae Soo Shin are employees of IL-YANG Pharmaceutical Co., Ltd. The other authors have no conflicts of interest to disclose.
Electronic supplementary material
Supplementary Table 1
Demographics of study participants in each part (DOCX 17 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jin, B.H., Yoo, B.W., Park, J. et al. Pharmacokinetic drug interaction and safety after coadministration of clarithromycin, amoxicillin, and ilaprazole: a randomised, open-label, one-way crossover, two parallel sequences study. Eur J Clin Pharmacol 74, 1149–1157 (2018). https://doi.org/10.1007/s00228-018-2489-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2489-2