Summary
Purpose
Little is known about the informativeness of initial patient reports before they are reviewed by a pharmacovigilance centre (PVC). We aim to describe the patterns of patient adverse drug reaction (ADR) reporting in France and estimate the contribution of a review by a PVC assessor on the informativeness of these reports.
Methods
A retrospective study was conducted on patient reports between July 2011 and July 2015. Informativeness of 16 key elements of information (including drug start and end date, duration of treatment, time to onset and duration of the ADR, outcome, medical history and concomitant medication) was assessed in initial reports before and after review by a pharmacovigilance assessor.
Results
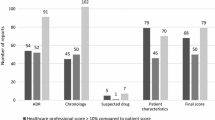
Overall, 240 reports concerning 522 ADR and involving 278 drugs were reported over this 4-year period. Mean number of available key elements of information in initial reports was increased from 11/16 to 15/16 after review of reports by the PVC. Time to onset and duration of the ADR were respectively available in only 51 and 58% of the reports before review compared to 83 and 90% after review. Medical history and concomitant medication were missing in 75% of the initial reports compared to less than 30% of the reports after review. Contacting the reporter enabled an increase of informativeness of most elements of information for more than 90% of the reports.
Conclusion
Patient reports often need to be completed on key elements of information that are required to assess reports. Both upstream education of patients and downstream intervention of a pharmacovigilance assessor to complete missing information could help to enhance the informativeness of such reports.



Similar content being viewed by others
References
Mitchell AS, Henry DA, Sanson-Fisher R, O’Connell DL (1988) Patients as a direct source of information on adverse drug reactions. BMJ 297(6653):891–893
Blenkinsopp A, Wilkie P, Wang M, Routledge PA (2007) Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol 63(2):148–156
Basch E (2010) The missing voice of patients in drug-safety reporting. N Engl J Med 362(10):865–869
van Grootheest K, de Jong-van den Berg L (2004) Patients’ role in reporting adverse drug reactions. Expert Opin Drug Saf 3(4):363–368
Mitchell AS, Henry D, Hennrikus D, O’Connell D (1994) Adverse drug reactions: can consumers provide early warnings ? Pharmacoepidemiol Drug Safety 3:257–264
Medawar C, Herxheimer A (2003) A comparison of adverse drug reaction reports from professionals and users, relating to risk of dependence and suicidal behaviour with paroxetine. Int J Risk Safety Med 16:5–19
Westin L, Albinson J (2008) 10 years of experiences with consumer reporting to KILEN—a Swedish consumer organisation. Drug Safety 31(Issue 10):885
Inch J, Watson MC, Anakwe-Umeh S (2012) Patient versus healthcare professional spontaneous adverse drug reaction reporting: a systematic review. Drug Saf 35(10):807–818
Hazell L, Cornelius V, Hannaford P, Shakir S, Avery AJ (2013) Yellow Card Study Collaboration. How do patients contribute to signal detection?: a retrospective analysis of spontaneous reporting of adverse drug reactions in the UK’s Yellow Card Scheme. Drug Saf 36(3):199–206
De Langen J, van Hunsel F, Passier A, de Jong-van den Berg L, van Grootheest K (2008) Adverse drug reaction reporting by patients in the Netherlands: three years of experience. Drug Saf 31(6):515–524
Avery AJ, Anderson C, Bond CM, Fortnum H, Gifford A, Hannaford PC et al (2011) Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow Card Scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess 15(20):1–234 iii-iv
Edwards IR, Lindquist M, Wiholm BE, Napke E (1990) Quality criteria for early signals of possible adverse drug reactions. Lancet 336(8708):156–158
Nasrallah-Irles D, Castot A, Thomas L, Babai S, Delorme B, Le-Louët H (2008) Adverse drug reactions: a pilot study on patient reporting through patient associations. Therapie 63(5):385–392
Rolfes L, van Hunsel F, Wilkes S, van Grootheest K, van Puijenbroek E (2015) Adverse drug reaction reports of patients and healthcare professionals-differences in reported information. Pharmacoepidemiol Drug Saf 24(2):152–158
Härmark L, van Hunsel F, Grundmark B (2015) ADR reporting by the general public: lessons learnt from the Dutch and Swedish systems. Drug Saf 38(4):337–347
Inácio P, Cavaco A, Airaksinen M (2017) Value of patient reporting to the pharmacovigilance system: a systematic review. Br J Clin Pharmacol 83(2):227–246
Vial T (2016) Therapie. French pharmacovigilance. Missions, organization and perspectives 71(2):143–150
Schjøtt J (2017 Jan) Benefits of a national network of drug information centres: RELIS. Eur J Clin Pharmacol 73(1):125–126
EMA (2012). Guideline on good pharmacovigilance practices (GVP) Module VI–management and reporting of adverse reactions to medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/02/WC500123203.pdf. Accessed 15 August 2016
Rolfes L, Wilkes S, van Hunsel F, van Puijenbroek E, van Grootheest K (2014 Jun) Important information regarding reporting of adverse drug reactions: a qualitative study. Int J Pharm Pract 22(3):231–233
WHO collaboration centre for drug statistics methodology (2016). ATC classification. http://www.whocc.no/atc_ddd_index/. Accessed 3 May 2016.
Medical Dictionary for Regulatory Activities (2016). http://www.meddra.org. Accessed 10 June 2016.
EMA (1995). ICH Topic E2A. Step 5: Note for guidance on clinical safety data management: definitions and standards for expedited reporting. June 1995. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002749.pdf. Accessed 12 June 2016.
Begaud B, Evreux JC, Jouglard J et al (1985) Imputation of the unexpected or toxic effects of drugs: actualization of the method used in France. Therapie 40(2):111–118
Miremont-Salamé G, Théophile H, Haramburu F, Bégaud B (2016) Causality assessment in pharmacovigilance: the French method and its successive updates. Therapie 71(2):179–186
ANSM (2016). Bulletin des vigilances Avril 2016. ANSM. http://ansm.sante.fr/var/ansm_site/storage/original/application/3973b7ec970dc261c5aae0357550dfa2.pdf. Accessed 10 August 2016.
Anderson C, Krska J, Murphy E, Avery A (2011 Nov) The importance of direct patient reporting of suspected adverse drug reactions: a patient perspective. Br J Clin Pharmacol 72(5):806–822
Härmark L, Lie-Kwie M, Berm L, de Gier H, van Grootheest K (2013 Jan) Patients’ motives for participating in active post-marketing surveillance. Pharmacoepidemiol Drug Saf 22(1):70–76
Aagaard L, Nielsen LH, Hansen EH (2009) Consumer reporting of adverse drug reactions: a retrospective analysis of the Danish adverse drug reaction database from 2004 to 2006. Drug Saf 32(11):1067–1074
van Hunsel F, Passier A, van Grootheest AC (2009) Comparing patients’ and healthcare professionals’ ADR reports after media attention. The broadcast of a Dutch television programme about the benefits and risks of statins as an example. Br J Clin Pharmacol 67(5):558–564
McLernon DJ, Bond CM, Hannaford PC, Watson MC, Lee AJ, Hazell L, al. Yellow Card Collaboration (2010) Adverse drug reaction reporting in the UK: a retrospective observational comparison of yellow card reports submitted by patients and healthcare professionals. Drug Saf 33(9):775–788
Durrieu G, Palmaro A, Pourcel L, Caillet C, Faucher A, Jacquet A et al (2012) First French experience of ADR reporting by patients after a mass immunization campaign with influenza A (H1N1) pandemic vaccines: a comparison of reports submitted by patients and healthcare professionals. Drug Saf 35(10):845–854
AFSSAPS (2011). Expérience des signalements des effets indésirables par les patients. Assise du médicament. http://www.sante.gouv.fr/IMG/pdf/Agence_francaise_de_securite_sanitaire_des_produits_de_sante_AFSSAPS___Experiences_des_signalements_des_effets_indesirables_par_les_patients.pdf Accessed 2 August 2016.
Margraff F, Bertram D (2014) Adverse drug reaction reporting by patients: an overview of fifty countries. Drug Saf 37(6):409–419
Bergvall T, Norén GN, Lindquist M (2014) Vigi grade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf 37(1):65–77
Vilhelmsson A, Svensson T, Meeuwisse A, Carlsten A (2012) Experiences from consumer reports on psychiatric adverse drug reactions with antidepressant medication: a qualitative study of reports to a consumer association. BMC Pharmacol Toxicol 13:19
Medicines and Healthcare products Regulatory Agency (MHRA) (2015). Digital evolution for ground-breaking Yellow Card Scheme. https://www.gov.uk/government/news/digital-evolution-for-ground-breaking-yellow-card-scheme. Accessed 16 June 2016.
Uppsala Monitoring Centre (2016). Take and Tell program. http://www.takeandtell.org. Accessed 16 August 2016.
Author information
Authors and Affiliations
Contributions
F Kheloufi drafted the manuscript and participated to the interpretation of data. J Ponte-Astoul, M Boyer, D Laugier-Castellan, B Rodrigues and F Rouby participated in the assessment of ADR reports. MJ Jean-Pastor, J Micallef, F Rouby and O Blin participated in the interpretation of data, writing and correction of the article. All listed authors were involved in revision of the draft for important intellectual content and provided final approval for manuscript submission.
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kheloufi, F., Default, A., Rouby, F. et al. Informativeness of patient initial reports of adverse drug reactions. Can it be improved by a pharmacovigilance centre?. Eur J Clin Pharmacol 73, 1009–1018 (2017). https://doi.org/10.1007/s00228-017-2254-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2254-y