Abstract
Objective
To study the effects of berberine (BBR) on the blood concentration and pharmacokinetics of cyclosporin A (CsA) in renal-transplant recipients.
Methods
In a randomized and controlled clinical trial, 52 renal-transplant recipients were treated with CsA and 0.2 g BBR three times daily for 3 months, while another 52 subjects received CsA without BBR co-administration. Blood trough concentration of CsA and biochemistry indexes for hepatic and renal functions were determined. For the pharmacokinetic study, six renal-transplant recipients were included with a 3-mg/kg dosage of CsA twice daily before and after oral co-administration of 0.2 g BBR three times daily for 12 days.
Results
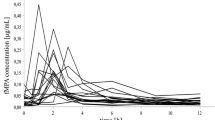
The trough blood concentrations and the ratios of concentration/dose of CsA in the BBR-treated group increased by 88.9% and 98.4%, respectively, compared with those at baseline (P<0.05). As for the BBR-free group, they rose by 64.5% and 69.4%, respectively, relative to those at baseline (P<0.01). Nevertheless, the final blood concentrations and the ratios of concentration/dose of CsA in BBR-treated patients were still 29.3% and 27.8%, respectively, higher than those in BBR-free patients (P<0.05). No significant effects on liver or renal functions were observed under coadministration of BBR. After co-administration of BBR in six patients for 12 days, the mean AUC of CsA was increased by 34.5% (P<0.05). The mean time taken to reach the peak blood concentration (tmax) and the mean half-life (t1/2) of CsA were increased by 1.7 h and 2.7 h, respectively (P<0.05). The average percentage increases in the steady-state drug concentration (Css) and minimum blood concentration (Cmin) were 34.5% and 88.3%, respectively (P<0.05). In addition, the average percentage decrease in CL/F was 40.4% (P<0.05) and the peak-to-through fluctuation index was significantly reduced (P<0.01).
Conclusion
The BBR can markedly elevate the blood concentration of CsA in renal-transplant recipients in both clinical and pharmacokinetic studies. This combination may allow a reduction of the CsA dosage. The mechanism for this interaction is most likely explained by inhibition of CYP3A4 by BBR in the liver and/or small intestine.

Similar content being viewed by others

References
Martin JE, Daoud AJ, Schroeder TJ, First MR (1999) The clinical and economic potential of cyclosporin drug interactions. Pharmacoeconomics 15:317–337
Jones TE (1997) The use of other drugs to allow a lower dosage of cyclosporin to be used. Therapeutic and pharmacoeconomic considerations. Clin Pharmacokinet 32:357–367
Asberg A, Christensen H, Hartman A, Carlson E, Molden E, Berg KJ (1999) Pharmacokinetic interaction between microemulsion formulated cyclosporine and diltiazem in renal transplanted recipients. Eur J Clin Pharmacol 55:383–387
Gomez DY, Wacher VJ, Tomlanovich SJ, Hebert ME, Benet LZ (1995) The effects of ketoconazole on the intestinal metabolism and bioavailability of cyclosporine. Clin Pharmacol Ther 58:15–19
Freeman DJ, Martell R, Carruthers SG, Heinrichs D, Keown PA, Stiller CR (1987) Cyclosporin-erythromycin interaction in normal subjects. Br J Clin Pharmacol 23:776–778
Hughes CM, Swanton JG, Collierr PS (1993) Cyclosporin A and erythromycin: a study of a drug interaction in the situ perfused rat liver model. Biopharm Drug Dispos 14:615–625
Keogh A, Sprant P, McCosker C, Macdonald P, Mundy J, Kaan A (1995) Ketoconazole to reduce the need for cyclosporine after cardiac transplantation. N Engl J Med 333:628–633
Jones TE, Morris RG, Mathew TH (1997) Diltiazem-cyclosporin pharmacokinetic interaction—dose-response relationship. Br J Clin Pharmacol 44:499–504
Yee GC, Stanley DL, Pessa LJ, Dalla Costa T, Beltz SE, Ruiz J, Lowenthal DT (1995) Effect of grapefruit juice on blood cyclosporin concentration. Lancet 345:955–956
Proppe DG, Hoch OD, McLean AJ, Visser KE (1995) Influence of chronic ingestion of grapefruit juice on steady state blood concentration of cyclosporine A in renal transplant patients with stable graft function. Br J Clin Pharmacol 39:337–338
Jantova S, Cipak L, Cernakova M, kost‘alova D (2003) Effects of berberine on proliferation, cell cycle and apoptosis in Hela and L1210 cells. J Pharm Pharmacol 55:1143–1149
Zeng XH, Zeng XJ, Li YY (2003) Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 92:173–176
Pan GY, Huang ZJ, Wang GJ, Fawcett JP, Liu XD, Zhao XC, Sun JG, Xie YY (2003) The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med 69:632–636
Kong WJ, Wei J, Abidi P, Lin MH, Inaba S, Li C, Wan YL, Wang ZZ, Si SY, Pan HN, Wang SK, Wu JD, Wang Y, Li ZR, Liu JW, Jiang JD (2004) Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 10:1344–1351
Wu XC, Xin HW, Li Q, Yu AR, Zhong MY, Tang K (2003) Pharmacokinetics of cyclosporine A coadministrated with berberine in Chinese healthy volunteers. Chin J Hosp Pharm 23:707–709
Li Q, Wu XC, Xin HW, Yu AR, Zhong MY, Leng JH, Jiang ZJ, Zhu M, Tang LG, Xie S (2001) Clinical study on coadministration of cyclosporin A and berberine hydrochloride in renal transplanted recipients. Chin J Clin Pharmacol 17:114–117
Awni WM, Kasiske BL, Heim-Duthoy K, Rao KV (1989) Long-term cyclosporine pharmacokinetic changes in renal transplant recipients: effects of binding and metabolism. Clin Pharmacol Ther 45:41–48
Wilms HW, Straeten V, Lison AE (1988) Different pharmacokinetics of cyclosporine A early and late after renal transplantation. Transpl Proc 20(2 Suppl 2):481–484
Xin HW, Wu XC, Li Q, Zhong MY, Yu AR, Zhu M (2002) Effects of berberine hydrochloride and coadministration with cyclosporine A on liver microsomal cytochrome P450 isoenzyme in mice. Chin Pharm J 37:496–499
Xin HW, Wu XC, Li Q, Yu AR, Zhong MY, Zhu M, Liu YY (2002) Effects of coadministration of berberine chloride with cyclosporin on liver microsomal cytochrome P450 isoenzyme and mdr1 in rats. Chin Pharmacol Bull 18:397–401
Chatterjee P, Franklin MR (2003) Human cytochrome P450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug Metab Dispos 31:1311–1391
Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY (2002) The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol 91:193–197
Maeng HJ, Yoo HJ, Kim IW, Song IS, Chung SJ, Shim CK (2002) P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J Pharm Sci 91:2614–2621
Lin HL, Liu TY, Lui WY, Chi CW (1999) Up-regulation of multidrug resistance transporter expression by berberine in human and murine hepatoma cells. Cancer 85:1937–1942
He L, Liu GQ (2002) Effects of various principles from Chinese herbal medicine on rhodamine123 accumulation in brain capillary endothelial cells. Acta Pharmacol Sin 23:591–596
Patton PR, Brunson ME, Pfaff WW, Howard RJ, Peterson JC, Ramos EL, Karlix JL (1994) A preliminary report of diltiazem and ketoconazole. Their cyclosporine-sparing effect and impact on transplant outcome. Transplantation 57:889–892
Pichard L, Fabre I, Daujat M, Domergue J, Joyeux H, Maurel P (1992) Effect of Corticosteroids on the expression of cytochromes P450 and on cyclosporin A oxidase activity in primary cultures of human hepatocytes. Mol Pharmacol 41:1047–1055
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Li, Q., Xin, H. et al. Effects of berberine on the blood concentration of cyclosporin A in renal transplanted recipients: clinical and pharmacokinetic study. Eur J Clin Pharmacol 61, 567–572 (2005). https://doi.org/10.1007/s00228-005-0952-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0952-3