Abstract
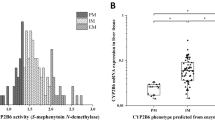
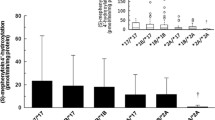
Several-fold differences have been observed among patients in the biotransformation of cyclophosphamide. The aim of this study was to investigate the contribution of CYP2C9 and CYP2C19 and their polymorphisms to the variability of cyclophosphamide activation. The formation of 4-hydroxycyclophosphamide was studied in microsomes from a total of 32 different genotyped human livers, as well as in yeast microsomes expressing different genetic variants of CYP2C9 and CYP2C19. The kinetic data obtained in the yeast system revealed that the intrinsic clearance (Vmax/Km) of cyclophosphamide by CYP2C9.2 and CYP2C9.3 samples was approximately threefold lower than that by CYP2C9.1. However, in liver microsomes, there were no statistically significant differences in the intrinsic clearance of 4-hydroxycyclophosphamide formation between the group of seven CYP2C9*1/*1 livers and the remaining nine with one or two variant CYP2C9 alleles (P>0.7). We found a statistically significant correlation (r s=0.65, P=0.003) between 4-hydroxylation of cyclophosphamide and 5′-hydroxylation of R-omeprazole, a measure of CYP2C19 activity in human liver microsomes (n=19). No correlation was found between 4-hydroxylation of cyclophosphamide and the formation rate of hydroxycelecoxib, mainly catalysed by CYP2C9 (r s=0.17, P=0.55, n=32). In conclusion, based on the correlation with the formation of R-5′-hydroxyomeprazole, CYP2C19 may partly contribute to the bioactivation of cyclophosphamide in human liver microsomes, while the role of CYP2C9 appears minor.



Similar content being viewed by others
References
Brock N (1989) Oxazaphosphorine cytostatics: past-present-future. Seventh Cain memorial award lecture. Cancer Res 49:1–7
Clarke L, Waxman DJ (1989) Oxidative metabolism of cyclophosphamide: identification of the hepatic monooxygenase catalysts of drug activation. Cancer Res 49:2344–2350
Boddy AV, Yule SM (2000) Metabolism and pharmacokinetics of oxazaphosphorines. Clin Pharmacokinet 38:291–304
Roy P, Yu LJ, Crespi CL, Waxman DJ (1999) Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P450 profiles. Drug Metab Dispos 27:655–666
Chang TK, Weber GF, Crespi CL, Waxman DJ (1993) Differential activation of cyclophosphamide and ifosphamide by cytochromes P450 2B and 3A in human liver microsomes. Cancer Res 53:5629–5637
Chang TK, Yu L, Goldstein JA, Waxman DJ (1997) Identification of the polymorphically expressed CYP2C19 and the wild-type CYP2C9-ILE359 allele as low-Km catalysts of cyclophosphamide and ifosfamide activation. Pharmacogenetics 7:211–221
Ren S, Yang JS, Kalhorn TF, Slattery JT (1997) Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res 57:4229–4235
Huang Z, Roy P, Waxman DJ (2000) Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem Pharmacol 59:961–972
Hassan M, Nilsson C, Olsson H, Lundin J, Osterborg A (1999) The influence of interferon-alpha on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite in patients with multiple myeloma. Eur J Haematol 63:163–170
Hassan M, Ljungman P, Ringden O, Hassan Z, Oberg G, Nilsson C, Bekassy A, Bielenstein M, Abdel-Rehim M, Georen S, Astner L (2000) The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant 25:915–924
Slattery JT, Kalhorn TF, McDonald GB, Lambert K, Buckner CD, Bensinger WI, Anasetti C, Appelbaum FR (1996) Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol 14:1484–1494
Bertilsson L (1995) Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet 29:192–209
Alvan G, Bertilsson L, Dahl ML, Ingelman-Sundberg M, Sjoqvist F (2001) Moving toward genetic profiling in patient care: the scope and rationale of pharmacogenetic/ecogenetic investigation. Drug Metab Dispos 29:580–585
Aithal GP, Day CP, Kesteven PJ, Daly AK (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 353:717–719
Meyer UA (2000) Pharmacogenetics and adverse drug reactions. Lancet 356:1667–1671
Miners JO, Birkett DJ (1998) Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45:525–538
Scordo MG, Aklillu E, Yasar Ü, Dahl ML, Spina E, Ingelman-Sundberg M (2001) Genetic polymorphism of cytochrome P450 2C9 in a Caucasian and a black African population. Br J Clin Pharmacol 52:447–450
Ingelman-Sundberg M (2001) Implications of polymorphic cytochrome P450-dependent drug metabolism for drug development. Drug Metab Dispos 29:570–573
de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA (1994) The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 269:15419–15422
Goldstein JA, de Morais SM (1994) Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics 4:285–299
Tang C, Shou M, Rushmore T, Mei Q, Sandhu P, Woolf E, Rose M, Gelmann A, Greenberg H, De Lepeleire I, Van Hecken A, De Schepper P, Ebel D, Schwartz J, Rodrigues D (2001) In-vitro metabolism of celecoxib, a cyclooxygenase-2 inhibitor, by allelic variant forms of human liver microsomal cytochrome P450 2C9: correlation with CYP2C9 genotype and in-vivo pharmacokinetics. Pharmacogenetics 11:223–235
Tang C, Shou M, Mei Q, Rushmore TH, Rodrigues AD (2000) Major role of human liver microsomal cytochrome P450 2C9 (CYP2C9) in the oxidative metabolism of celecoxib, a novel cyclooxygenase-II inhibitor. J Pharmacol Exp Ther 293:453–459
Chang M, Dahl ML, Tybring G, Gotharson E, Bertilsson L (1995) Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics 5:358–363
Tybring G, Bottiger Y, Widen J, Bertilsson L (1997) Enantioselective hydroxylation of omeprazole catalyzed by CYP2C19 in Swedish white subjects. Clin Pharmacol Ther 62:129–137
Yasar Ü, Tybring G, Hidestrand M, Oscarson M, Ingelman-Sundberg M, Dahl ML, Eliasson E (2001) The role of CYP2C9 polymorphism in losartan oxidation. Drug Metab Dispos 29:1051–1056
Oscarson M, Hidestrand M, Johansson I, Ingelman-Sundberg M (1997) A combination of mutations in the CYP2D6*17 (CYP2D6Z) allele causes alterations in enzyme function. Mol Pharmacol 52:1034–1040
Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM, Miners JO, Birkett DJ, Goldstein JA (1996) The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 6:341–349
Yasar Ü, Eliasson E, Dahl ML, Johansson I, Ingelman-Sundberg M, Sjoqvist F (1999) Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun 254:628–631
Griskevicius L, Meurling L, Hassan M (2002) A simple and accurate method using fluorescent detection for determination of 4-hydroxycyclophosphamide in plasma. Ther Drug Monit 24:405–409
Sandberg M, Yasar Ü, Strömberg P, Höög J-O, Eliasson E (2002) Oxidation of celecoxib by polymorphic cytochrome P450 2C9 and alcohol dehydrogenase. Br J Clin Pharmacol 54:423–429
Mirghani RA, Yasar U, Zheng T, Cook JM, Gustafsson LL, Tybring G, Ericsson O (2002) Enzyme kinetics for the formation of 3-hydroxyquinine and three new metabolites of quinine in vitro; 3-hydroxylation by CYP3A4 is indeed the major metabolic pathway. Drug Metab Dispos 30:1368–1371
Moore MJ (1991) Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 20:194–208
Andersson T, Regardh CG, Dahl-Puustinen ML, Bertilsson L (1990) Slow omeprazole metabolizers are also poor S-mephenytoin hydroxylators. Ther Drug Monit 12:415–416
Andersson T, Regardh CG, Lou YC, Zhang Y, Dahl ML, Bertilsson L (1992) Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics 2:25–31
Abelo A, Andersson TB, Antonsson M, Naudot AK, Skanberg I, Weidolf L (2000) Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos 28:966–972
Rettie AE, Korzekwa KR, Kunze KL, Lawrence RF, Eddy AC, Aoyama T, Gelboin HV, Gonzalez FJ, Trager WF (1992) Hydroxylation of warfarin by human cDNA-expressed cytochrome P450: a role for P4502C9 in the etiology of (S)-warfarin-drug interactions. Chem Res Toxicol 5:54–59
Acknowledgements
The study was granted by Swedish Children Cancer Foundation (PROJ01/59), Medical Research Council (3902, 4496 and 5949), Stockholm Cancer Foundation (02:119), King Gustav V Jubilee Foundation and Karolinska Institutet.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Griskevicius, L., Yasar, Ü., Sandberg, M. et al. Bioactivation of cyclophosphamide: the role of polymorphic CYP2C enzymes. Eur J Clin Pharmacol 59, 103–109 (2003). https://doi.org/10.1007/s00228-003-0590-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0590-6