Abstract
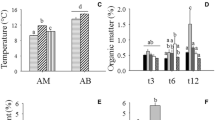
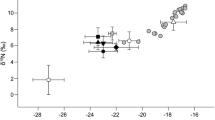
Sandy beaches constitute a dynamic interface between marine and terrestrial environments, where inflows of organic matter occur mainly in the form of beach-cast wrack. Decomposition and processing of these inputs stand out as central components of ecosystem functioning and the biogeochemical processes associated with nutrient and carbon cycling. To investigate the effect of upper-beach macrofauna on wrack decomposition and carbon cycling, a manipulative experiment was carried out on Ladeira beach (42°34.33″N; 9°3.16″W—NW Spain) and ran for 43 days from 1 June 2011, where wrack decomposition rates and metabolism in the presence or absence of invertebrate macrofauna were assessed. The results showed that activities carried out by macrofauna inhabiting the upper beach are relevant in the decomposition of wrack deposits. Both the weight losses and the degradation rates were significantly higher in treatments with macrofauna access. CO2 fluxes measured throughout the experiment showed that wrack patches act as ‘hot spots’ of biogeochemical activity, supporting higher metabolic rates with maximum values of CO2 flux recorded in this study ~ 12 µmol C m−2 s−1. Microbial activity was estimated as being the main contributor to the respiration measured during wrack decay, but fragmentation and activities carried out by the macrofauna had significant effects on the mass loss, degradation rates and carbon fluxes associated with wrack decay process.







Similar content being viewed by others
References
Barreiro F, Gómez M, Lastra M, López J, de la Huz R (2011) Annual cycle of wrack supply to sandy beaches: effect of the physical environment. Mar Ecol Prog Ser 433:65–74. https://doi.org/10.3354/meps09130
Barton PS, Cunningham SA, Lindenmayer DB, Manning AD (2013) The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 171:761–772. https://doi.org/10.1007/s00442-012-2460-3
Colombini I, Chelazzi L (2003) Influence of marine allochthonous input on sandy beach communities. Oceanogr Mar Biol Annu Rev 41:115–159
Colombini I, Aloia A, Fallaci M, Pezzoli G, Chelazzi L (2000) Temporal and spatial use of stranded wrack by the macrofauna of a tropical sandy beach. Mar Biol 136:531–541
Coupland GT, Duarte CM, Walker DI (2007) High metabolic rates in beach cast communities. Ecosystems 10:1341–1350. https://doi.org/10.1007/s10021-007-9102-3
Cronin G, Hay ME (1996) Susceptibility to herbivores depends on recent history of both the plant and the animal. Ecology 77:1531–1543
Davies RI (1971) Relation of polyphenols to decomposition of organic matter and to pedogenetic processes. Soil Sci 111:80–85
del Giorgio PA, Williams PJ (2005) Respiration in aquatic ecosystems. Oxford University Press, Oxford
Dobson T (1974) Studies on the biology of the kelp-fly Coelopa in Great Britain. J Nat Hist 8:155–177
Dubois A, Iken K (2012) Seasonal variation in kelp phlorotannins in relation to grazer abundance and environmental variables in the Alaskan sublittoral zone. Algae 27:9–19. https://doi.org/10.4490/algae.2012.27.1.009
Dugan JE, Hubbard DM, McCrary MD, Pierson MO (2003) The response of macrofauna communities and shorebirds to macrophyte wrack subsidies on exposed sandy beach of southern California. Estuar Coast Shelf Sci 58:25–40
Duggins DO, Simenstad CA, Estes JA (1989) Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245:170–173
Duong HLS (2008) Investigating the ecological implications of wrack removal on South Australian sandy beaches. PhD Thesis, Flinders University, South Australia
Eereveld P, Hübner L, Schaefer G, Zimmer M (2013) Herbivory on macro-algae affects colonization of beach-cast algal wrack by detritivores but not its decomposition. Oceanologia 55:339–358. https://doi.org/10.5697/oc.55-2.339
Emery K (1961) A simple method of measuring beach profiles. Limnol Oceanogr 6:90–93
Gómez M, Barreiro F, López J, Lastra M, de la Huz R (2013) Deposition patterns of algal wrack species on estuarine beaches. Aquat Bot 105:25–33. https://doi.org/10.1016/j.aquabot.2012.12.001
Gonçalves SC, Marques JC (2011) The effects of season and wrack subsidy on the community functioning of exposed sandy beaches. Estuar Coast Shelf Sci. https://doi.org/10.1016/j.ecss.2011.08.036
Griffiths C, Stenton-Dozey JME (1981) The fauna and rate of degradation of stranded kelp. Estuar Coast Shelf Sci 12:645–653
Griffiths CL, Stenton-Dozey JME, Koop K (1983) Kelp wrack and the flow of energy through a sandy beach ecosystem. In: McLachlan A, Erasmus T (eds) Sandy beaches as ecosystems. Junk, The Hague, pp 547–556
Hay ME, Fenical W (1996) Chemical ecology and marine biodiversity: insights and products from the sea. Oceanography 9:10–20
Inglis G (1989) The colonisation and degradation of stranded Macrocystis pyrifera (L.) C. Ag. by the macrofauna of a New Zealand sandy beach. J Exp Mar Biol Ecol 125:203–217
Jaramillo E, De la Huz MR, Duarte C, Contreras H (2006) Algal wrack deposits and macroinfaunal arthropods on sandy beaches of the Chilean coast. Rev Chil Hist Nat 79:337–351
Jędrzejczak MF (2002a) Stranded Zostera marina L. vs wrack fauna community interactions on a Baltic sandy beach (Hel, Poland): a short-term pilot study. Part I. Driftline effects of fragmented detritivory, leaching and decay rates. Oceanology 44:273–286
Jędrzejczak MF (2002b) Stranded Zostera marina L. vs wrack fauna community interactions on a Baltic sandy beach (Hel, Poland): a short-term pilot study. Part II. Driftline effects of succession changes and colonisation of beach fauna. Oceanologia 44:37–387
Lastra M, Page HM, Dugan JE, Hubbard DM, Rodil IF (2008) Processing of allochthonous macrophyte subsidies by sandy beach consumers: estimates of feeding rates and impacts on food resources. Mar Biol 154:163–174
Lastra M, López J, Neves G (2015) Algal decay, temperature and body size influencing trophic behaviour of wrack consumers in sandy beaches. Mar Biol 162:221–233. https://doi.org/10.1007/s00227-014-2562-z
Lavoie DR (1985) Population dynamics and ecology of beach wrack macroinvertebrates of the central California coast. Bull South Calif Acad Sci 84:1–22
Lewis T, Mews M, Jelinski D, Zimmer M (2007) Detrital subsidy to the supratidal zone provides feeding habitat for intertidal crabs. Estuar Coast 30:451–458
Loreau M, Holt R (2004) Spatial flows and the regulation of ecosystems. Am Nat 163:606–615
Loreau M, Mouquet N, Holt RD (2003) Meta-ecosystems: a theoretical framework for a spatial ecosystem ecology. Ecol Lett 6:673–679. https://doi.org/10.1046/j.1461-0248.2003.00483.x
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Ca Johnston, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312. https://doi.org/10.1007/s10021-003-0161-9
McLachlan A, Brown AC (2006) The ecology of sandy shores. Elsevier, Amsterdam
Mews M, Zimmer M, Jelinski D (2006) Species-specific decomposition rates of beach-cast wrack in Barkley Sound, British Columbia, Canada. Mar Ecol Prog Ser 328:155–160. https://doi.org/10.3354/meps328155
Migné A, Davoult D, Spilmont N, Menu D, Boucher G, Gattuso JP, Rybarczyk H (2002) A closed-chamber CO2-flux method for estimating intertidal primary production and respiration under emersed conditions. Mar Biol 140:865–869. https://doi.org/10.1007/s00227-001-0741-1
Moore JC, Berlow EL, Coleman DC, Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ, Nadelhoffer K, Rosemond AD, Post DM, Sabo JL, Scow KM, Vanni MJ, Wall DH (2004) Detritus, trophic dynamics and biodiversity. Ecol Lett 7:584–600. https://doi.org/10.1111/j.1461-0248.2004.00606.x
Niemeck RA, Mathieson AC (1976) An ecological study of Fucus spiralis. J Exp Mar Biol Ecol 24:33–48
Norderhaug KM, Fredriksen S, Nygaard K (2003) Trophic importance of Laminaria hyperborea to kelp forest consumers and the importance of bacterial degradation to food quality. Mar Ecol Prog Ser 255:135–144
Ochieng CA, Erftemeijer PL (1999) Accumulation of seagrass beach cast along the Kenyan coast: a quantitative assessment. Aquat Bot 65:221–238
Olabarria C, Lastra M, Garrido J (2007) Succession of macrofauna on macroalgal wrack of an exposed sandy beach: effects of patch size and site. Mar Environ Res 63:19–40. https://doi.org/10.1016/j.marenvres.2006.06.001
Polis GA, Hurd SD (1996) Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am Nat 147:396–423
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of ecology: the dynamics food webs subsidized spatially. Annu Rev Ecol Evol Syst 28:289–316
Poore AGB, Hill NA (2006) Sources of variation in herbivore preference: among-individual and past diet effects on amphipod host choice. Mar Biol 149:1403–1410. https://doi.org/10.1007/s00227-006-0307-3
Robertson A, Hansen JA (1982) Decomposing seaweed: a nuisance or a vital link in coastal food chains?. CSIRO Marine Laboratories, Cronulla
Rodil IF, Lastra M, López J (2007) Macroinfauna community structure and biochemical composition of sedimentary organic matter along a gradient of wave exposure in sandy beaches (NW Spain). Hydrobiologia 579:301–316. https://doi.org/10.1007/s10750-006-0443-2
Rodil IF, Cividanes S, Lastra M, López J (2008a) Seasonal variability in the vertical distribution of benthic macrofauna and sedimentary organic matter in an estuarine beach (NW Spain). Estuar Coast 31:382–395. https://doi.org/10.1007/s12237-007-9017-4
Rodil IF, Olabarria C, Lastra M, Lopez J (2008b) Differential effects of native and invasive algal wrack on macrofaunal assemblages inhabiting exposed sandy beaches. J Exp Mar Biol Ecol 358:1–13
Rodil IF, Lucena-Moya P, Olabarria C, Arenas F (2015) Alteration of macroalgal subsidies by climate-associated stressors affects behavior of wrack-reliant beach consumers. Ecosystems 18:428–440. https://doi.org/10.1007/s10021-014-9836-7
Rodríguez JG (2004) Community structure of intertidal meiofauna along a gradient of morphodynamic states on an exposed North Sea beach. Sarsia 89:22–32
Rossi F, Underwood A (2002) Small-scale disturbance and increased nutrients as influences on intertidal macrobenthic assemblages: experimental burial of wrack in different intertidal environments. Mar Ecol Prog Ser 242:29–39
Schlesinger WH (1977) Carbon balance in terrestrial detritus. Annu Rev Ecol Syst 8:51–81. https://doi.org/10.1146/annurev.es.08.110177.000411
Spiller DA, Piovia-Scorr J, Wright AN, Yang LH, Takimoto G, Schoener TW, Iwata T (2010) Marine subsidies have multiple effects on coastal food webs. Ecology 91:1424–1434
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Studies in ecology. University of California Press, Berkeley
Targett NM, Arnold TM (1998) Minireview—predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J Phycol 34:195–205. https://doi.org/10.1046/j.1529-8817.1998.340195.x
Urban-Malinga B, Burska D (2009) The colonization of macroalgal wrack by the meiofauna in the Arctic intertidal. Estuar Coast and Shelf Sci 85:666–670
Van Alstyne KL (1995) Comparison of three methods for quantifying brown algal polyphenolic compounds. J Chem Ecol 21:45–58
Van Alstyne KL, McCarthy JJ III, Hustead CL, Duggins DO (1999) Geographic variation in polyphenolic levels of Northeastern Pacific kelps and rockweeds. Mar Biol 133:371–379. https://doi.org/10.1007/s002270050476
Wilson J, Buchsbaum R, Valiela I, Swain T (1986) Decomposition in salt marsh ecosystems: phenolic dynamics during decay of litter of Spartina alterniflora. Mar Ecol Prog Ser 29:177–187. https://doi.org/10.3354/meps029177
Acknowledgements
The authors thank J. Pascual and L. Gestoso for help with experiment placement and fieldwork, and L. Palacios for support in the macrofauna identification process. We would like to thank to two anonymous referees for suggestions and improvements of the manuscript. Thanks are also due to I. Emmett for proofreading the English revision of this manuscript.
Funding
This research was supported by the Xunta de Galicia (Regional Autonomous Government of Galicia—GRC 2013-004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained.
Additional information
Responsible Editor: M. Huettel.
Reviewed by Ch. L. Griffiths and K. Meyer-Arendt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gómez, M., Barreiro, F., López, J. et al. Effect of upper beach macrofauna on nutrient cycling of sandy beaches: metabolic rates during wrack decay. Mar Biol 165, 133 (2018). https://doi.org/10.1007/s00227-018-3392-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3392-1