Abstract
Bycatch is one of the main threats affecting marine megafauna worldwide, not only because of its prevalence, but also because the impact of high levels of bycatch in small oceanic regions may spread over whole oceans due to the complex dispersal patterns of bycaught species. Here, we use intrinsic and genetic markers to understand the impact of bycatch on the Atlantic and Mediterranean populations of the loggerhead turtle sharing the same foraging grounds in the western Mediterranean Sea. Turtles of Atlantic origin settle on the continental shelf later and at a larger size than turtles of Mediterranean origin and hence have been suggested to be more vulnerable to pelagic fishing gears, whereas those of Mediterranean origin would be more vulnerable to neritic ones. To assess whether this hypothesis holds true, we compared the genetic make-up of turtle bycatch from drifting longlines and bottom trawl/trammel nets in three different regions (eastern mainland Spain, southern Balearic Islands and southern Italy). A total of 176 incidentally caught turtles were considered, and size and habitat use, as revealed by stable isotopes, were incorporated to the analysis. No genetic, size or isotopic differences were found between turtles caught with drifting longlines and bottom trawl/trammel nets within any of the three regions. However, genetic, size and isotopic differences were detected among regions, regardless of the fishing gear. Thus, the population make-up of loggerhead bycatch depends on the area where the fishing operations are conducted, but not on the fishing gear used. Accordingly, the actual impact of loggerhead bycatch in the Mediterranean Sea will depend not only on the total number of turtles taken, but also on the geographic distribution of the fishing effort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fishing activity is a well-known threat to many endangered marine species because of a combination of overfishing and habitat disturbance (Pauly et al. 2005). However, fisheries not only may affect targeted species, but also un-targeted species, threatened through overfishing of food resources or lethal direct interactions. Bycatch, the unintentional capture of non-targeted species during fishing operations, has been described as one of the most important factors causing the decline of marine species worldwide, especially of large marine vertebrates: birds, sharks, marine mammals and sea turtles (Lewison et al. 2004). The vulnerability of many large marine vertebrate populations to fisheries interactions is also accentuated by their long lifespan, late age at maturity and low reproductive output (Heppell et al. 1999).
In order to assess the impact of bycatch on marine megafauna, it is crucial to evaluate the number of animals incidentally caught and to estimate the demographic effects of such catch rates for each of the threatened populations (Bache 2003). However, spatial division among populations is not always clear, particularly as these species usually occur in remote oceanic habitats, are distributed across entire oceans and often have complex life cycles involving migrations that cover thousands of kilometres (Anderson et al. 2013). Thus, assignment of caught individuals to specific populations often cannot be carried out based on geographical divisions, and the need to use different approaches such as the use of genetic markers arises (Bowen et al. 1995; Edwards et al. 2001).
Sea turtles present complex life cycles involving several habitat shifts and long-distance migrations (Plotkin 2003). Loggerhead turtles (Caretta caretta), for instance, spend several years in the open ocean during early life and recruit to neritic habitats as late juveniles or immatures (Bolten 2003), although, in some populations, a number of adults may remain oceanic throughout their entire lives (Watanabe et al. 2011; Eder et al. 2012). Sea turtle bycatch has been of strong concern among scientists for decades (Hamann et al. 2010), especially considering that most sea turtles are listed as endangered under the IUCN Red List of Threatened Species. Among all fisheries, drifting longlines targeting large pelagic fish (Lewison and Crowder 2007) and bottom trawling in neritic areas (Casale 2011; Wallace et al. 2013) have been the focus of most of the research on turtle bycatch due to the large number of captures worldwide. Mid-water trawls have also been identified as relevant threats to sea turtle populations in certain areas (Lewison and Crowder 2007). However, due to the implementation of turtle excluder devices in mid-water trawls (particularly in prawn fisheries) in many countries, bycatch numbers associated with this type of gear have plummeted down to 90 % (northern Australia; Robins et al. 2002).
The foraging grounds in the Mediterranean Sea are used by juvenile loggerhead turtles of Atlantic and Mediterranean origin (Carreras et al. 2006; Clusa et al. 2014), and hence, turtle bycatch in this area may have broad implications across the North Atlantic and Mediterranean nesting stocks (Álvarez de Quevedo et al. 2013). Pioneering research on the origin of the loggerhead turtles using the Mediterranean foraging grounds indicated that drifting longlines captured a mixture of turtles of Atlantic and Mediterranean origin, whereas bottom trawling captured only turtles of Mediterranean origin (Laurent et al. 1998). Unfortunately, it is unclear whether that pattern was real and emerged from the earlier settlement of Mediterranean turtles into neritic habitats (Casale et al. 2008; Piovano et al. 2011) or was an artefact caused by the eastward decreasing abundance of turtles of Atlantic origin within the Mediterranean Sea (Maffucci et al. 2006; Clusa et al. 2014). This is extremely relevant to the conservation of loggerhead turtles because each scenario leads to a different management strategy, focused either on fishing gear types or on fishing grounds.
To disentangle whether fishing ground and/or fishing gear has a differential impact on turtles from different origins present in the Mediterranean as juveniles, we collected samples of loggerheads caught by drifting longline and bottom trawling/trammel nets within three areas (eastern mainland Spain, southern Balearic Islands and southern Italy). These areas corresponded to three sub-basins differing in salinity, sea surface temperature, primary productivity and surface circulation (Millot 1999; Bosc et al. 2004). We measured the size of bycaught turtles, and through the use of stable isotope ratios and genetic mitochondrial and nuclear DNA markers, we assessed whether juvenile loggerhead turtles caught with different gears in the same region consistently differ (1) in size, (2) in patterns of habitat use and (3) in haplotype frequencies and natal origin. With this approach, we aimed to assess the impact that different fishing gears on different foraging grounds might have on Atlantic and Mediterranean rookeries.
Materials and methods
Bycatch sampling
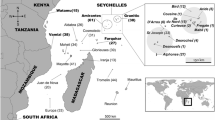
A total of 176 juvenile loggerhead turtles incidentally caught from 2001 to 2012 in three major regions of the western and central Mediterranean (Fig. 1) were sampled: 55 from eastern mainland Spain (SPA), 85 from southern Balearic Islands (BAL) and 36 from southern Italy (SIT). Muscle samples were collected from dead animals and stored in 95 % ethanol, whilst blood samples were taken from live animals and stored frozen. Live animals sampled were flipper-tagged to make sure that each individual was only sampled once.
Turtles were divided into two groups for each region, according to bycatch source: turtles caught by drifting longline (DLL) and turtles caught by trawl and trammel fisheries (TWL). Samples were collected over 10 years (Supplementary Table 1), and even if the type of gear and fishing effort (number of boats and hours at sea) might have changed during this period, these did not affect the results and main conclusions of this study. This was shown by the lack of significant differences between two groups on the distribution of number of samples per group analysed through time (sign test; Z = 0.917, P = 0.359).
Genetic characterisation of loggerhead turtle bycatch
DNA from bycaught turtles (N = 176) was extracted with the QIAamp extraction kit (QIAGEN®) and sequenced to assign their natal origin (Atlantic or Mediterranean). A fragment of the mtDNA control region was amplified by polymerase chain reaction (PCR) using the primer pairs TCR1–TCR2 (short fragment; Norman et al. 1994) and LCM15382-H950 (long fragment; Abreu-Grobois et al. 2006) following the protocols described in Carreras et al. (2006) and Clusa et al. (2013), respectively. The samples amplified by the primer pairs TCR1–TCR2 came from previous surveys, and hence, no long fragments could be amplified due to sample degradation. All samples were sequenced in forward and reverse directions to confirm variable sites on both strands of DNA on an ABI 3730 automated DNA analyser.
Sequences were manually aligned with BioEdit v7.2.5 (Hall 1999) and compared to the short (380bp) and long (815bp) haplotypes previously described for the species, compiled at the Archie Carr Center for Sea Turtle Research of the University of Florida (ACCSTR; http://accstr.ufl.edu).
Individual assignments to natal origin were undertaken following the sequential method described in Carreras et al. (2011). Whenever an individual carried an mtDNA haplotype exclusive to an Atlantic or Mediterranean nesting area, this individual was assumed to have originated from the corresponding nesting area, without any further analysis. Individuals carrying shared haplotypes, orphan haplotypes (not described in any nesting area to date), or individuals that failed to amplify for the long mtDNA fragment were genotyped at seven microsatellite loci. Control individuals with known origin carrying exclusive haplotypes were tested by Carreras et al. (2011) with both techniques, and controls were correctly reassigned when genotyped. This sequential approach highly reduced the analysis cost.
Seven microsatellites had previously been used in C.caretta: Cc117, Cm72, Cm84 and Ei8 (FitzSimmons et al. 1995); Cc7 and Cc141 (FitzSimmons et al. 1996); and Ccar176 (Moore and Ball 2002; modified by Carreras et al. 2007). These individuals were assigned with STRUCTURE v2.3.4 (Pritchard et al. 2000) by comparison with the baseline individual genotypes used in Carreras et al. (2011). Origin assignments were only considered valid when the probability of belonging to the Atlantic or Mediterranean stock was higher than 0.7 for one of the two groups.
Overall differences in origin frequencies (Atlantic or Mediterranean) between fishing gears among the three sampled regions (Fig. 1) were assessed with an analysis of molecular variance (AMOVA) using ARLEQUIN v3.5 (Excoffier and Lischer 2010). The same program was used to estimate genetic differentiation between groups of juveniles caught with different fishing gears in each sampled region. Based on short mtDNA control region sequences, pairwise F ST values were computed and its significance was assessed with an exact test.
Size characterisation of loggerhead turtle bycatch
Curved carapace length notch-to-tip (CCL n−t ) was measured for all individuals. Mediterranean turtles only smaller than 69 cm CCL n−t were used for size and isotopic comparisons of the juvenile stage, as this is the mean minimum size of nesting females in the Mediterranean (Margaritoulis et al. 2003). On the contrary, all turtles assigned to an Atlantic origin were used for subsequent analyses, as Atlantic turtles start nesting at sizes much larger (87–104 cm CCL n−t ; Turtle Expert Working Group 2009) than the ones recorded in this study. A two-way ANCOVA, with gear and region as fixed factors and year as a covariate, was used to test for differences in size (CCL n−t ) of turtles caught by the different fishing gears in three regions analysed. In this study, year was always chosen as a covariate according to its independence from the other two factors (gear and region) and the fact that year was not different across the groups as revealed by the sign test above (Miller and Chapman 2001).
Stable isotope characterisation of loggerhead turtle bycatch
Ethanol-preserved muscle samples from 11 juvenile individuals from each fishing gear and region were thoroughly rinsed with distilled water (periodical changes of water over 12 h) to avoid the presence of ethanol—even if this has minimal effects on stable isotope analyses (Arrington and Winemiller 2002). Subsequently, samples were oven-dried at 60 °C for 48–72 h. They were ground into fine powder, and lipids were extracted from all tissues with a chloroform–methanol (2:1) solution. This was undertaken as lipids are depleted in 13C in comparison with other molecules, and hence, changes in the lipid contents of the sample may bias the δ 13C values (Logan and Lutcavage 2008).
Approximately 0.3 mg of each dry and powdered sample was combusted at 1000 °C. The analyses were performed in a continuous-flow isotope ratio mass spectrometer (Flash 112 IRMS Delta C Series EA Thermo Finnigan) at Serveis Científics i Tecnològics at the University of Barcelona. Stable isotope ratios were expressed in the following delta notation (δ) in parts per thousand (‰):
where X is 13C or 15N and R is the corresponding ratio of the heavier to the lighter isotope (i.e. 13C/12C or 15N/14N). International isotope standards of known 13C/12C and 15N/14N ratios were used to a precision of 0.2 ‰: IAEA CH6 (δ 13C = −10.3 ‰), USGS 40 (δ 13C = −25.8 ‰) and IAEA CH7 (δ 13C = −31.6 ‰) for carbon and USGS 40 (δ 15N = −4.3 ‰), IAEA N1 (δ 15N = +0.8 ‰), IAEA 600 (δ 15N = +1.0 ‰) and IAEA N2 (δ 15N = +20.4 ‰) for nitrogen.
A two-way ANCOVA, considering gear and region as fixed factors and sampling year as a covariate, was used to test for statistical differences in the nitrogen and carbon stable isotope ratios of turtles caught by the different fishing gears in three regions analysed. Relationships between size and isotopic signatures of bycaught individuals were assessed with Pearson’s correlation tests in three regions analysed.
Results
Genetic characterisation of loggerhead turtle bycatch
Individual assignment through the amplification of mtDNA and nDNA allowed us to assign 153 individuals (87 %) to their natal stocks (Atlantic or Mediterranean origin), a similar successful assignment ratio to previous studies (Carreras et al. 2011). Of these, short mtDNA control region haplotypes allowed assigning the origin of 47 individuals. Whilst only one individual carried an exclusive Mediterranean short mtDNA haplotype (CC-A6), the remaining 46 assigned individuals carried exclusive Atlantic haplotypes: CC-A1 (39 individuals); CC-A5 (1); CC-A6 (1); CC-A10 (1); CC-A14 (4). The rest of analysed individuals carried haplotypes found in both Atlantic and Mediterranean rookeries (CC-A2, CC-A3, CC-A20) and hence could not be assigned with just short mtDNA haplotypes. Furthermore, in regards to the long mtDNA haplotypes, these allowed the assignment of another 12 individuals, all carrying the CC-A2.9 haplotype—only found in Mediterranean rookeries until the moment (Clusa et al. 2013). A total of 94 individuals were further assigned with microsatellite markers, but the remaining 23 individuals (13 %) could not be assigned due to low assigning probabilities mostly caused by amplification failure of some of the microsatellite loci.
Juvenile individuals of Atlantic and Mediterranean origin were not homogeneously distributed among groups (Fig. 2). Marginal differences were detected among regions (AMOVA; percentage of variation = 33.54 %, P = 0.062), but there were no statistically significant differences between fishing gears within each region (AMOVA; percentage of variation = 2.91 %, P = 0.181). The exact tests revealed that pairwise genetic distances between fishing gears within the same region were not significant, and the only significant differences were detected between the southern Balearic Islands and the other two regions regardless of fishing gear (Table 1). Juvenile turtles of Atlantic origin caught in the southern Balearic Islands represented 87.8 % of the turtle bycatch of drifting longlines and 81.8 % of the turtle bycatch of bottom trawls/trammel in the same area. Conversely, the proportion of juvenile turtles of Atlantic origin was always lower than 65 % both for drifting longlines and for trammel nets/bottom trawls off eastern mainland Spain and southern Italy (Fig. 2).
Individual assignment results showing the percentage of juvenile individuals incidentally caught in the studied regions (SPA: eastern mainland Spain, BAL: southern Balearic Islands, SIT: southern Italy) by drifting longlines (DLL) and trawling/trammel nets (TWL) from Mediterranean (dark grey) and Atlantic (light grey) rookeries. The proportion of unassigned individuals is shown in white
Size characterisation of loggerhead turtle bycatch
The size of turtles captured with drifting longlines (Fig. 3) ranged between 25 cm (in SIT) and 76 cm (in BAL), whilst those captured with trawling/trammel nets ranged between 22 cm (in SIT) and 80 cm (in SIT).
No significant differences in size were observed between fishing gears nor for the interaction between regions and fishing gears (Table 2). However, significant size differences were found among regions, with turtles caught in southern Italy being significantly smaller than turtles caught in eastern mainland Spain and southern Balearic Islands (Fig. 3), as assessed by a post hoc Tukey’s test (P = 0.031 and P < 0.01, respectively).
Stable isotope characterisation of loggerhead turtle bycatch
Sampling year did not affect the values of δ 13C, which did not differ among fishing gears or regions (Table 2; Fig. 4). Likewise, sampling year did not affect the δ 15N values, which were significantly different among regions, but not between fishing gears (Table 2). The post hoc Tukey’s test revealed much lower δ 15N values for the turtles caught in the central Mediterranean (SIT) than for those captured in the western Mediterranean, but no differences between eastern mainland Spain and southern Balearic Islands (Fig. 4). Nevertheless, there was a significant interaction term because in southern Italy turtles caught with drifting longlines were more enriched in 15N than turtles caught with bottom trawls/trammel nets, whereas the opposite was true for eastern mainland Spain and southern Balearic Islands. No correlation was found between the size of an individual and its δ 13C and δ 15N values in any of the three studied regions analysed (Pearson’s correlations, all P > 0.05).
Stable isotope composition of juvenile loggerhead turtles from the regions analysed. Samples pooled by fishing gear (light grey: drifting longline; dark grey: trawling/trammel net) and region (triangles: eastern mainland Spain; circles: southern Balearic Islands; squares: southern Italy). Sample size is 11 turtles for each category
Discussion
The complex dispersal patterns of many marine predators sometimes hinder the understanding of the broad significance of high levels of bycatch in small geographic areas. The western Mediterranean has supported some of the highest sea turtle bycatch levels worldwide since the 1980s (Lewison et al. 2004; Wallace et al. 2013), and its potential significance for sea turtle conservation is much larger than suggested simply by its geographic extension, as loggerhead turtles from two different regional management units are regularly bycaught there (Laurent et al. 1998; Carreras et al. 2006, 2011; Clusa et al. 2014). The same might be true for other marine predators with complex, non-univocal migratory connections between nesting and foraging grounds (e.g. Gómez-Díaz and González-Solís 2007; Valenzuela et al. 2009; Sequeira et al. 2013).
Our results revealed no significant differences in size, stable isotopic ratios or origin of the juvenile loggerhead turtles caught with drifting longlines and bottom trawling/trammel nets within any of the three regions considered, although differences existed among regions. This indicates that vulnerability to fishing gear does not differ between turtles of Atlantic and Mediterranean origin inhabiting any given region. Accordingly, all the fisheries that incidentally catch juvenile sea turtles in the Mediterranean Sea (Casale 2011) may potentially impact both the Northwest Atlantic and Mediterranean management units. Thus, the actual impact on each management unit will depend on the total number of turtles taken and the geographic distribution of the catch within the Mediterranean, but not on the composition of the fleets involved. This is because of the heterogeneous composition of loggerhead turtle stocks within the Mediterranean Sea (Carreras et al. 2006, 2011; Maffucci et al. 2006; Clusa et al. 2014). Thus, the differences in the genetic characterisation of longline and trawling bycatch previously reported by Laurent et al. (1998) might have emerged because fishing gears were regionally nested, and hence, the gear and region factors were confounded.
The absence of differences in the size and stable isotope ratios of loggerhead turtles caught by drifting longlines and neritic gears off southern Balearic Islands and eastern mainland Spain revealed that turtles behave similarly in each area regardless of their natal origin. These results strongly corroborate previous satellite telemetry data that suggested that juvenile turtles inhabiting the southern Balearic Islands spend most of the time in oceanic waters and only occasionally visit the continental shelf (Cardona et al. 2005; Revelles et al. 2007b). Similarly, in southern Italy, tracked small and large juvenile turtles regularly moved between oceanic and neritic habitats (Bentivegna 2002). On the other hand, significant differences were found among areas for δ 15N values. The lower values observed off southern Italy are consistent with the eastward pattern of particulate organic matter within the Mediterranean Sea (Pantoja et al. 2002), and the results found in three regions fit within the values found for other marine vertebrates of the Mediterranean Sea (Pinnegar and Polunin 2000; Navarro et al. 2011). The eastward pattern found, combined with the turnover rate of stable isotopes in muscle (Reich et al. 2008), is congruent with a limited exchange of turtles between adjoining basins on a monthly scale, previously suggested by tagging (Revelles et al. 2008) and satellite telemetry (Bentivegna 2002; Cardona et al. 2005; Revelles et al. 2007b). The Algerian basin has been described as a hot spot for Atlantic juveniles, with a decreasing relative abundance of turtles of Atlantic origin from the Strait of Gibraltar to the Adriatic Sea (Carreras et al. 2006; Maffucci et al. 2006; Clusa et al. 2014). The presence of turtles of Atlantic origin in the western Mediterranean would also explain the individual size differences found among areas. Turtles bycaught in eastern mainland Spain and southern Balearic Islands are significantly larger than those from southern Italy probably because turtles of Atlantic origin are forced to stay in the area and grow until they are large enough to overcome the currents in the Alboran Sea and the Strait of Gibraltar to settle in the western Atlantic (Bowen et al. 2005; Revelles et al. 2007a).
Relatively lower proportions of turtles assigned to Mediterranean nesting areas were found in the sample sets of eastern mainland Spain and southern Italy in comparison with the proportion expected by mixed stock analyses. Former mixed stock analyses from the same area estimated a presence of 80–90 % of turtles of Mediterranean origin (Carreras et al. 2006) against the 50 % found through individual assignment (Carreras et al. 2011; present work). This is likely a consequence of the high proportion of unassigned individuals in both regions and the lower probability of assignment of turtles of Mediterranean origin as compared to those of Atlantic origin. Haplotype CC-A1.1 is the most frequent haplotype in Atlantic rookeries and is also exclusively from that area (Shamblin et al. 2014). Conversely, haplotype CC-A2.1 might be the most common in Mediterranean rookeries (Clusa et al. 2013), but is also shared with Atlantic rookeries. Thus, the power of assignment is higher on foraging grounds with a high proportion of turtles carrying CC-A1.1 than for foraging grounds with a high proportion of turtles carrying CC-A2.1 (the most abundant haplotype in eastern mainland Spain and southern Italy). As a consequence, a higher proportion of unassigned individuals are expected when turtles of Mediterranean origin are common as most Mediterranean individuals carry shared haplotypes. This hypothesis is supported by the congruence found between direct assignment and mixed stock analysis on the proportion of turtles of Atlantic origin (Clusa et al. 2014).
Genetic tools have been previously used to track the origin of specific individuals to certain populations for management and conservation purposes of multiple species (Manel et al. 2005; Schwartz et al. 2007; Benestan et al. 2015). However, the results here presented highlight the need to use multiple markers not only in loggerhead turtle research, but also in all megafauna studies in general. In the case of the loggerhead turtle, the majority of previous studies that analysed short mtDNA haplotypes found inconclusive results due to lower resolution capacity and high numbers of shared haplotypes between different rookeries (Maffucci et al. 2006, Carreras et al. 2007, Casale et al. 2008, Saied et al. 2012). Thus, by using a combination of mtDNA and nuclear markers, there has been a remarkable increase in the power of individual assignment in this study. Even if the use of microsatellite markers in sea turtle research has been slowly increasing during this decade (Carreras et al. 2007, 2011; Monzón-Argüello et al. 2008; Dutton et al. 2013; Garofalo et al. 2013, Naro-Maciel et al. 2014), there is still an over-dominance of studies that only focus on mtDNA analyses. Accordingly, the re-analysis of turtles from certain areas with primers that amplify for longer fragments, together with the use of multiple microsatellite markers, would be highly recommendable. This would help increase the resolution of genetic differentiation not only among rookeries, but also among foraging grounds. Only by having a complete knowledge on the genetic structuring of nesting areas will individual assignments to natal origin be successful.
Implications for future conservation
The heterogeneous presence of individuals of Atlantic and Mediterranean origin in Mediterranean bycatch, as found in the present study, highlights that bycatch impact will strongly depend on turtle distribution, spatio-temporal overlap with fishing activities and mortality associated with each fishing gear. Casale (2011) estimated that over 132,000 sea turtles are caught every year in the Mediterranean Sea, of which 44,000 (the majority loggerhead turtles) are killed due to fatal interactions with fisheries. However, post-release mortality has been assessed for drifting longlines only recently (Álvarez de Quevedo et al. 2013), and nothing is known about other fishing gears.
Drifting longlines have been defined as the most threatening of all fishing gears for juvenile loggerhead turtles in the Mediterranean Sea (Deflorio et al. 2005), mainly impacting areas of Spain, Morocco, Tunisia, Italy, Greece and Libya (Jribi et al. 2008; Casale 2011). The estimated bycatch rate is of approximately 70,000 turtles caught per year (Casale 2011), and the mortality rate is approximately 35 % (Álvarez de Quevedo et al. 2013). However, that figure is probably not up to date, as the total number of turtles bycaught annually by the Spanish fleet has decreased from some 20,000 in the early 1990s to 6000 in 2006–2007 (Álvarez de Quevedo et al. 2014; Báez et al. 2014) and less than 1000 in recent years (Báez, personal communication). No recent figures exist for the rest of the Mediterranean to our knowledge. In any case, the Spanish fleet used to be the one with the highest rate of loggerhead longline bycatch (Casale 2011), and the current study reveals that Spain longliners might capture mainly turtles of Atlantic origin, thus potentially impacting primarily on the populations nesting in south Florida. Conversely, the Italian fleet might capture a larger proportion of turtles of Mediterranean origin, as this fleet operates primarily in the Ionian Sea (Deflorio et al. 2005; Casale 2011) where the prevalence of turtles of Mediterranean origin is higher (Clusa et al. 2014).
On another front, countries involved in trawling activities are Spain, Italy, Tunisia, Croatia, Greece, Turkey, Egypt and Libya (Lazar and Tvrtkovic 1995; Oruç 2001; Álvarez de Quevedo et al. 2010; Casale 2011; Domènech et al. 2014) with a particular presence in the northern Adriatic Sea (Lazar and Tvrtkovic 1995; Casale et al. 2004). Even if bycatch derived from trawling activities has been considered less of a threat than that coming from longlines because of its lower bycatch rates in the Mediterranean Sea (39,000 captures per year; Casale 2011), it should not be ignored. According to Wallace et al. (2013), on a global scale mortality rates associated with trawling (0.26 ± 0.29) and set nets (0.32 ± 0.3) are significantly higher than in longlines (0.07 ± 0.19), although rates vary depending on fishing depth (from 0.01 ± 0.01 in surface/drift longlines to 0.54 ± 0.32 in bottom set nets), and little is known about post-release mortality rates (Álvarez de Quevedo et al. 2013; García-Párraga et al. 2014). Accordingly, fishery interactions can represent a significant threat for sea turtle populations (Lewison et al. 2004; Lewison and Crowder 2007), and there is a growing need to understand the effects of bycatch on wild populations. However, this can become a difficult subject as reliable data on fishing effort and bycatch quantification in some areas are irregular or scarce.
In the current study, even if the analysed fishing gears caught turtles of both Atlantic and Mediterranean origin, we can conclude that the impact that fisheries may have on wild populations will differ depending on the foraging grounds used by each of these populations, even if the analysed gears caught turtles of both Atlantic and Mediterranean origin. This is shown by the lack of differentiation between turtles caught with drifting longlines and trawl/trammel nets within each region but the existence of composition differentiation among regions. Accordingly, the type of fishing gear used in each region determines the mortality rate of the caught turtles and the susceptibility of being caught, but does not determine the origin of its individuals.
With hatchlings and juvenile turtles using foraging grounds located in faraway areas from their rookeries, insufficiency of focusing all the conservation measures in nesting beaches comes to light. Further research should focus on determining the composition of each foraging ground, as proper identification of the affected populations is essential. Thus, the use of multiple intrinsic and genetic marker characterisation of bycatch should be implemented in new conservation plans as populations with distinct origins and conservation statuses may share the same foraging grounds.
References
Abreu-Grobois A, Horrocks J, Formia A, Dutton P, LeRoux R, Vélez-Zuazo X, Soares L, Meylan P (2006)New mtDNA Dloop primers which work for a variety of marine turtle species may increase the resolution capacity of mixed stock analysis. In: Proceedings of the Annual Symposium on Sea Turtle Biology Conservation p 179
Álvarez de Quevedo I, Cardona L, De Haro A, Pubill E, Aguilar A (2010) Sources of bycatch of loggerhead sea turtles in the western Mediterranean other than drifting longlines. ICES J Mar Sci 67:677–685
Álvarez de Quevedo I, San Félix M, Cardona L (2013) Mortality rates in by-caught loggerhead turtle Caretta caretta in the Mediterranean Sea and implications for the Atlantic populations. Mar Ecol Prog Ser 489:225–234
Álvarez de Quevedo I, San Félix M, Cardona L (2014) Temporal trends in the by-catch of loggerhead turtles Caretta caretta in the Mediterranean Sea: replay to Báez et al. Mar Ecol Prog Ser 504:303–304
Anderson JJ, Gurarie E, Bracis C, Burke BJ, Laidre KL (2013) Modeling climate change impacts on phenology and population dynamics of migratory marine species. Ecol Model 264:83–97
Arrington DA, Winemiller KO (2002) Preservation effects on stable isotope analysis of fish muscle. Trans Am Fish Soc 131:337–342
Bache SJ (2003) Bycatch mitigation tools: selecting fisheries, setting limits, and modifying gear. Ocean Coast Manag 46:103–125
Báez JC, García Barcelona S, Real R, Macías D et al (2014) Estimating by-catch of loggerhead turtles in the Mediterranean: comment on Álvarez de Quevedo et al. Mar Ecol Prog Ser 504:301–302
Benestan L, Gosselin T, Perrier C, Sainte-Marie B, Rochette R (2015) RAD genotyping reveals fine-scale genetic structuring and provides powerful population assignment in a widely distributed marine species, the American lobster (Homarus americanus). Mol Ecol 24:3299–3315
Bentivegna F (2002) Intra-mediterranean migration of loggerhead sea turtles monitored by satellite telemetry. Mar Biol 141:795–800
Bolten AB (2003) Active swimmers-passive drifters: the oceanic juvenile stage of loggerheads in the Atlantic system. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtle. Smithsonian Books, Washington, pp 63–78
Bosc E, Bricaud A, Antoine D (2004) Seasonal and interannual variability in algal biomass and primary production in the Mediterranean Sea, as derived from 4 years of SeaWiFS observations. Glob Biogeochem Cycles 18:1005
Bowen BW, Abreu-Grobois FA, Balazs GH, Kamezaki N, Limpus CJ, Ferl RJ (1995) Trans-Pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial DNA markers. PNAS 92:3731–3734
Bowen BW, Bass AL, Soares L, Toonen RJ (2005) Conservation implications of complex population structure: lessons from the loggerhead turtle (Caretta caretta). Mol Ecol 14:2389–2402
Cardona L, Revelles M, Carreras C, San Félix M, Gazo M, Aguilar A (2005) Western Mediterranean immature loggerhead turtles: habitat use in spring and summer assessed through satellite tracking and aerial surveys. Mar Biol 147:583–591
Carreras C, Pont S, Maffucci F, Pascual M, Barceló A, Bentivegna F, Cardona L, Alegre F, SanFélix M, Fernández G, Aguilar A (2006) Genetic structuring of immature loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea reflects water circulation patterns. Mar Biol 149:1269–1279
Carreras C, Pascual M, Cardona L, Aguilar A, Margaritoulis D, Rees A, Turkozan O, Levy Y, Gasith A, Aureggi M, Khalil M (2007) The genetic structure of the loggerhead sea turtle (Caretta caretta) in the Mediterranean as revealed by nuclear and mitochondrial DNA and its conservation implications. Conserv Genet 8:761–775
Carreras C, Pascual M, Cardona L, Marco A, Bellido JJ, Castillo JJ, Tomás J, Raga JA, SanFélix M, Fernández G, Aguilar A (2011) Living together but remaining apart: Atlantic and Mediterranean loggerhead sea turtles (Caretta caretta) in shared feeding grounds. J Hered 102:666–677
Casale P (2011) Sea turtle by-catch in the Mediterranean. Fish Fish 12:299–316
Casale P, Laurent L, De Metrio G (2004) Incidental capture of marine turtles by the Italian trawl fishery in the north Adriatic Sea. Biol Conserv 119:287–295
Casale P, Abbate G, Freggi D, Conte N, Oliveiro M, Argano R (2008) Foraging ecology of loggerhead sea turtles Caretta caretta in the central Mediterranean Sea: evidence for a relaxed life history model. Mar Ecol Prog Ser 372:265–276
Clusa M, Carreras C, Pascual M, Demetropoulos A, Margaritoulis D, Rees AF, Hamza AA, Khalil M, Aureggi M, Levy Y, Türkozan O, Marco A, Aguilar A, Cardona L (2013) Mitochondrial DNA reveals Pleistocenic colonisation of the Mediterranean by loggerhead turtles (Caretta caretta). J Exp Mar Biol Ecol 439:15–24
Clusa M, Carreras C, Pascual M, Gaughran SJ, Piovano S, Giacoma C, Fernández G, Levy Y, Tomás J, Raga JA, Maffucci F, Hochscheid S, Aguilar A, Cardona L (2014) Fine-scale distribution of juvenile Atlantic and Mediterranean loggerhead turtles (Caretta caretta) in the Mediterranean Sea. Mar Biol 161:509–519
Deflorio M, Aprea A, Corriero A, Santamaria N, De Metrio G (2005) Incidental captures of sea turtles by swordfish and albacore longlines in the Ionian Sea. Fish Sci 71:1010–1018
Domènech F, Álvarez de Quevedo I, Merchán M, Revuelta O, Vélez-Rubio G, Bitón S, Cardona L, Tomás J (2014) Incidental catch of marine turtles by Spanish bottom trawlers in the western Mediterranean. Aquat Conserv. doi:10.1002/aqc.2463
Dutton PH, Roden SE, Stewart KR, LaCasella E, Tiwari M, Formia A, Thomé JC, Livingstone SR, Eckert S, Chacon-Chaverri D, Rivalan P, Allman P (2013) Population stock structure of leatherback turtles (Dermochelys coriacea) in the Atlantic revealed using mtDNA and microsatellite markers. Conserv Genet 14:625–636
Eder E, Ceballos A, Martins S, Pérez-García H, Marín I, Marco A, Cardona L (2012) Foraging dichotomy in loggerhead sea turtles Caretta caretta off northwestern Africa. Mar Ecol Prog Ser 470:113–122
Edwards SV, Silva MC, Friesen VL, Warheit KI (2001) Molecular genetic markers in the analysis of seabird bycatch populations. In: Melvin E, Parrish J (eds) Seabird bycatch: trends roadblocks and solutions. University of Alaska Sea Grant, Anchorage, pp 115–140
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10:564–567
FitzSimmons NN, Moritz C, Moore SS (1995) Conservation and dynamics of microsatellite loci over 300 million years of marine turtle evolution. Mol Biol Evol 12:432–440
FitzSimmons NN, Moritz C, Limpus CJ, Miller JD, Parmenter CJ, Prince RI (1996) Comparative genetic structure of green, loggerhead, and flatback population in Australia based on variable mtDNA and nDNA regions. In: Bowen BW, Witzell WN (eds) Proceedings of the International Symposium on Sea Turtle Conservation Genetics, NOAA Technical Memorandum NMFS-SEFSC-396, pp 25–32
García-Párraga D, Crespo-Picazo JL, Bernaldo de Quirós Y, Cervera V, Martí-Bonmati L, Díaz-Delgado J, Arbelo M, Moore MJ, Jepson PD, Fernández A (2014) Decompression sickness (‘the bends’) in sea turtles. Dis Aquat Org 111:191–205
Garofalo L, Mastrogiacomo A, Casale P, Carlini R, Eleni C, Freggi D, Gelli D, Knittweis L, Mifsud C, Mingozzi T, Novarini N, Scaravelli D, Scillitani G, Oliveiro M, Novelleto A (2013) Genetic characterization of central Mediterranean stocks of the loggerhead turtle (Caretta caretta) using mitochondrial and nuclear markers, and conservation implications. Aquat Conserv 23:868–884
Gómez-Díaz E, González-Solís J (2007) Geographic assignment of seabirds to their origin: combining morphologic, genetic, and biogeochemical analyses. Ecol Appl 17:1484–1498
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hamann M, Godfrey MH, Seminoff JA, Arthur K, Barata PCR, Bjorndal KA, Bolten A, Broderick AC, Campbell LM, Carreras C, Dutton PH, Epperly S, Fitzsimmons NN, Formia A, Girondot M, Hays GC, Jiunn CI, Kaska Y, Lewison R, Mortimer JA, Nichols WJ, Reina RD, Shanker K, Spotila JR, Tomás J, Wallace BP, Work TM, Zbinden JA, Godley BJ (2010) Global research priorities for sea turtles: informing management and conservation in the 21st century. Endanger Species Res 11:245–269
Heppell SS, Crowder LB, Menzel TR (1999) Life table analysis of long-lived marine species, with implications for conservation and management. In: Musick JA (ed) Life in the slow lane: ecology and conservation of long-lived marine animals. American Fisheries Society Symposium 23, Bethesda, pp 137–148
Jribi I, Echwikhi Bradai MN, Bouain A (2008) Incidental capture of sea turtles by longlines in the Gulf of Gabès (South Tunisia): a comparative study between bottom and surface longlines. Sci Mar 72:337–342
Laurent L, Casale P, Bradai MN, Godley BJ, Gerosa G, Broderick AC, Schroth W, Schierwater B, Levy AM, Freggi D, Abd El-Mawla EM, Hadoud DA, Gomati HE, Domingo M, Hadjichristophorou M, Kornaraky L, Demirayak F, Gautier CH (1998) Molecular resolution of marine turtle stock composition in fishery bycatch: a case study in the Mediterranean. Mol Ecol 7:1529–1542
Lazar B, Tvrtkovic N (1995) Marine turtles in the eastern part of the Adriatic Sea: preliminary research. Natura Croat 4:59–74
Lewison RL, Crowder LB (2007) Putting longline bycatch of sea turtles into perspective. Conserv Biol 21:79–86
Lewison RL, Crowder LB, Read AJ, Freeman SA (2004) Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol Evol 19:598–604
Logan JM, Lutcavage ME (2008) A comparison of carbon and nitrogen stable isotope ratios of fish tissues following lipid extractions with non-polar and traditional chloroform/methanol solvent systems. Rapid Commun Mass Spectrom 22:1081–1086
Maffucci F, Kooistra WHCF, Bentivegna F (2006) Natal origin of loggerhead turtles, Caretta caretta, in the neritic habitat off the Italian coasts, Central Mediterranean. Biol Conserv 127:183–189
Manel S, Gaggiotti OE, Waples RS (2005) Assignment methods: matching biological questions with appropriate techniques. Trends Ecol Evol 20:136–142
Margaritoulis D, Argano R, Baran I, Bentivegna F, Bradai MN, Camiñas JA, Casale P, De Metrio G, Demetropoulos A, Gerosa G, Godley BJ, Haddoud DA, Houghton J, Laurent L, Lazar B (2003) Loggerhead turtles in the Mediterranean Sea: present knowledge and conservation perspectives. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtle. Smithsonian Books, Washington, pp 175–198
Miller GA, Chapman JP (2001) Misunderstanding analysis of covariance. J Abnorm Psychol 110:40–48
Millot C (1999) Circulation in the Western Mediterranean Sea. J Mar Sys 20:423–442
Monzón-Argüello C, Muñoz J, Marco López-Jurado LF, Rico C (2008) Twelve new polymorphic microsatellite markers from the loggerhead sea turtle (Caretta caretta) and cross-species amplification on other marine turtle species. Conserv Genet 9:1045–1049
Moore MK, Ball RM Jr (2002) Multiple paternity in loggerhead turtle (Caretta caretta) nests on Melbourne Beach, Florida: a microsatellite analysis. Mol Ecol 11:281–288
Naro-Maciel E, Reid BN, Alter E, Amato G, Bjorndal KA, Bolten AB, Martin M, Nairn CJ, Shamblin B, Pineda-Catalan O (2014) From refugia to rookeries: Phylogeography of Atlantic green turtles. J Exp Mar Biol Ecol 461:306–316
Navarro J, Coll M, Louzao M, Palomera I, Delgado A, Forero MG (2011) Comparison of ecosystem modelling and isotopic approach as ecological tools to investigate food webs in the NW Mediterranean Sea. J Exp Mar Biol Ecol 401:97–104
Norman JA, Moritz C, Limpus CJ (1994) Mitochondrial DNA control region polymorphisms: genetic markers for ecological studies of marine turtles. Mol Ecol 3:363–373
Oruç A (2001) Trawl fisheries in the eastern Mediterranean and their impact on marine turtles. Zool Middle East 24:119–125
Pantoja S, Repeta DJ, Sachs JP, Sigman DM (2002) Stable isotope constraints on the nitrogen cycle of the Mediterranean Sea water column. Deep Sea Res Part I 49:1609–1621
Pauly D, Watson R, Alder J (2005) Global trends in world fisheries: impacts on marine ecosystems and food security. Philos T Roy Soc B 360:5–12
Pinnegar JK, Polunin NVC (2000) Contributions of stable-isotope data to elucidating food webs of Mediterranean rocky littoral fishes. Oecologia 122:399–409
Piovano S, Clusa M, Carreras C, Giacoma C, Pascual M, Cardona L (2011) Different growth rates between loggerhead sea turtles (Caretta caretta) of Mediterranean and Atlantic origin in the Mediterranean Sea. Mar Biol 158:2577–2587
Plotkin PT (2003) Adult migrations and habitat use. In: Lutz PL, Musick JA, Wyneken J (eds) The biology of sea turtles II. CRC Press, Boca Ratón, pp 225–241
Pritchard JK, Stephens M, Donelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Reich KJ, Bjorndal KA, Martínez del Rio C (2008) Effects of growth and tissue type on the kinetics of 13C and 15N incorporation in a rapidly growing ectotherm. Oecologia 155:651–663
Revelles M, Carreras C, Cardona L, Marco A, Bentivegna F, Castillo JJ, De Martino G, Mons JL, Smith MB, Rico C, Pascual M, Aguilar A (2007a) Evidence for an asymmetrical size exchange of loggerhead sea turtles between the Mediterranean and the Atlantic trough the Straits of Gibraltar. J Exp Mar Biol Ecol 349:261–271
Revelles M, Isern-Fontanet J, Cardona L, San Félix M, Carreras C, Aguilar A (2007b) Mesoscale eddies, surface circulation and the scale of habitat selection by immature loggerhead sea turtles. J Exp Mar Biol Ecol 347:41–57
Revelles M, Camiñas JA, Cardona L, Parga ML, Tomás J, Aguila A, Alegre F, Raga A, Bertolero A, Oliver G (2008) Tagging reveals limited exchange of immature loggerhead sea turtles (Caretta caretta) between regions in the western Mediterranean. Sci Mar 72:511–518
Robins CM, Goodspeed AM, Poiner I, Harch BD (2002) Monitoring the catch of turtles in the northern prawn fishery. Fisheries Research and Development Corporation Final Report. Department of Agriculture, Fisheries and Forestry, Canberra
Saied A, Maffucci F, Hochscheid S, Dryag S, Swayeb B, Borra M, Oureghi A, Procaccini G, Bentivegna F (2012) Loggerhead turtles nesting in Libya: an important management unit for the Mediterranean stock. Mar Ecol Prog Ser 450:207–218
Schwartz MK, Luikart G, Waples RS (2007) Genetic monitoring as a promising tool for conservation and management. Trends Ecol Evol 22:25–33
Sequeira AMM, Mellin C, Meekan MG, Sims DW, Bradshaw CJA (2013) Inferred global connectivity of whale shark Rhincodon typus populations. J Fish Biol 82:367–389
Shamblin BM, Bolten AB, Abreu-Grobois FA, Bjorndal KA, Cardona L, Carreras C, Clusa M, Monzón-Argüello C, Nairn CJ, Nielsen JT, Nel R, Soares LS, Stewart KR, Vilaça ST, Türkozan O, Yilmaz C, Dutton PH (2014) Geographic patterns of genetic variation in a broadly distributed marine vertebrate: New insights into loggerhead turtle stock structure from expanded mitochondrial DNA sequences. PLoS One 9:e85956
Turtle Expert Working Group (2009) An assessment of the loggerhead turtle population in the western north Atlantic Ocean. NOAA Tech Mem NMFS-SEFSC 575:1–131
Valenzuela LO, Sironi M, Rowntree VJ, Seger J (2009) Isotopic and genetic evidence for culturally inherited site fidelity to feeding grounds in southern right whales (Eubalaena australis). Mol Ecol 18:782–791
Wallace BP, Kot CY, DiMatteo AD, Lee T, Crowder LB, Lewison RL (2013) Impacts of fisheries bycatch on marine turtle populations worldwide: toward conservation and research priorities. Ecosphere 4: Article 40
Watanabe KK, Hatase H, Kinoshita M, Omuta K, Bando T, Kamezaki N, Sato K, Matsuzawa Y, Goto K, Nakashima Y, Takeshita H, Aoyama J, Tsukamoto K (2011) Population structure of the loggerhead turtle Caretta caretta, a large marine carnivore that exhibits alternative foraging behaviors. Mar Ecol Prog Ser 424:273–283
Acknowledgments
We are thankful to all the researchers, assistants and volunteers who collaborated in sample collection. This study was co-funded by projects CGL2009-10017, CTM2013-48163 and CGL2011-30413 of the Spanish Government (CICYT) and partially funded by the EU project Protección de Praderas de Posidonia en LICs de Baleares LIFE00NAT/E/7303 and Zoo de Barcelona. All the IRBio authors are part of the research groups 2014SGR-336 and 2014SGR-1364 of the Generalitat de Catalunya. The tissue samples used in this paper were provided by the BMA tissue bank managed by the Fundació Bosch Gimpera with the support of the Fundació pel Desenvolupament Sostenible. M. C. was supported by the Biodiversity Research Institute (IRBio) of the University of Barcelona and C. C. by the Beatriu de Pinós programme of the Generalitat de Catalunya. S. P. is supported by LIFE03 NAT/IT/000163 and LIFE04 NAT/IT/000187. J. T. And J. A. R. are also supported by and agreement with Conselleria d'Agricultura, Medi Ambient, Canvi Climàtic i Desenvolupament Rural and by project Prometeo (UV-CI-12-151) and PROMETEOII/2015/018 of the Generalitat Valenciana. We thank Fabiana Saporiti and Mónica Revelles for helpful aid with stable isotope protocols and Gregg Ashcroft for English grammar corrections. Map in Fig. 1 is a courtesy of Maptool (www.seaturtle.org).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: J. D. R. Houghton.
Reviewed by K. Stewart and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clusa, M., Carreras, C., Pascual, M. et al. Potential bycatch impact on distinct sea turtle populations is dependent on fishing ground rather than gear type in the Mediterranean Sea. Mar Biol 163, 122 (2016). https://doi.org/10.1007/s00227-016-2875-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2875-1