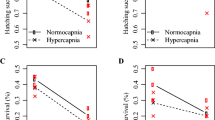
Abstract
The impact of a realistic warming scenario on the metabolic physiology of early cephalopod (squid Loligo vulgaris and cuttlefish Sepia officinalis) life stages was investigated. During exposure to the warming conditions (19 °C for the western coast of Portugal in 2100), the increase in oxygen consumption rates throughout embryogenesis was much steeper in squid (28-fold increase) than in cuttlefish (11-fold increase). The elevated catabolic activity–accelerated oxygen depletion within egg capsules, which exacerbated metabolic suppression toward the end of embryogenesis. Squid late-stage embryos appear to be more impacted by warming via metabolic suppression than cuttlefish embryos. At all temperature scenarios, the transition from encapsulated embryos to planktonic paralarvae implied metabolic increments higher than 100 %. Contrary to the nektobenthic strategy of cuttlefish newborns, the planktonic squid paralarvae rely predominantly on pulsed jet locomotion that dramatically increases their energy requirements. In the future, hatchlings will require more food per unit body size and, thus, feeding intake success will be crucial, especially for squid with high metabolic rates and low levels of metabolic reserves.






Similar content being viewed by others
References
Aitken JP, O’Dor RK (2004) Respirometry and swimming dynamics of the Australian cuttlefish Sepia apama. Mar Freshw Behav Physiol 37:217–234
Aitken JP, O’Dor RK, Jackson GD (2005) The secret life of the giant cuttlefish Sepia apama (Cephalopoda): behaviour and energetics in nature revealed through radio acoustic positioning and telemetry (RAPT). J Exp Mar Biol Ecol 320:77–91
Bartol IK, Krueger PS, Stewart WJ, Thompsonn JT (2009) Pulsed jet dynamics of squid hatchlings at intermediate Reynolds numbers. J Exp Biol 212:1506–1518
Boidron-Metairon IF (1995) Larval nutrition. In: Edward LMc (ed) Ecology of marine invertebrate larvae. CRC Press, Boca Raton, pp 223–248
Boletzky SV (1994) Embryonic development of cephalopods at low temperatures. Antarct Sci 6(2):139–142
Bouchaud O (1991) Energy consumption of the cuttlefish Sepia officinalis (Mollusca: Cephalopoda) during embryonic development, preliminary results. Bull Mar Sci 49(1–2):333–340
Boyle P, Rodhouse P (2005) Cephalopods: ecology and fisheries. Blackwell Science, Oxford 452 pp
Brante A (2006) An alternative mechanism to reduce intracapsular hypoxia in ovicapsules of Fusitriton oregonensis (Gastropoda). Mar Biol (Berl) 149:269–274
Brante A, Fernández M, Viard F (2008) Effect of oxygen conditions on intracapsular development in two calyptraeidae species with different modes of larval development. Mar Ecol Prog Ser 368:197–207
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Ocean Mar Biol Annu Rev 49:1–42
Cronin ER, Seymour RS (2000) Respiration of the eggs of the giant cuttlefish Sepia apama. Mar Biol 136:863–870
De Wachter B, Wolf G, Richard A, Decleir W (1988) Regulation of respiration during juvenile development of Sepia officinalis (Mollusca: Cephalopoda). Mar Biol 97:365–371
Denton EJ, Gilpin-Brown JB (1961a) The buoyancy of the cuttlefish, Sepia officinalis (L.). J Mar Biol Assess UK 41:319–342
Denton EJ, Gilpin-Brown JB (1961b) The effect of light on the buoyancy of the cuttlefish. J Mar Biol Assess UK 41:343–350
Fioroni P (1990) Our recent knowledge of the development of the cuttlefish (Sepia officinalis). Zool Anz 224:1–25
Frederich M, Pörtner HO (2000) Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am J Physiol 279:1531–1538
Gutowska MA, Melzner F (2009) Abiotic conditions in cephalopod (Sepia officinalis) eggs: embryonic development at low pH and high pCO2. Mar Biol 156:515–519
Hu MY, Sucré E, Charmantier-Daures M, Charmantier G, Lucassen M, Himmerkus N, Melzner F (2010) Localization of ion-regulatory epithelia in embryos and hatchlings of two cephalopods. Cell Tissue Res 339(3):571–583
Hu MY, Yung-Che T, Stumpp M, Gutowska MA, Kiko R, Lucassen M, Melzner F (2011) Elevated seawater PCO2 differentially affects branchial acid-base transporters over the course of development in the cephalopod Sepia officinalis. Am J Physiol Regul Integr Comp Physiol 301:R1700–R1709
Kamler E (1992) Early life history of fish: an energetic approach. Chapman and Hall, London
Katersky RS, Carter CG (2007) High growth efficiency occurs over a wide temperature range for juvenile barramundi Lates calcarifer fed a balanced diet. Aquaculture 272:444–450
Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser 373:275–284
Lemaire J (1971) Etude du développenaent embryonnaire de Sepia officinalis L., Thèse Doctorat 3 ème cycle. Université de Lille, France, p 70
Mangold-Wirz K (1963) Biologie des céphalopods benthiguis et nectonigues de la mer catalane. Vie Millieu 13(Suppl):285
Marvel MR, Fisher KC (1948) Developmental changes in the viability of squid embryos after subjection to cyanide. Biol Bull 94:45–54
McMahon JJ, Summers WC (1971) Temperature effects on the development rate of squid (Loligo pealei) embryos. Biol Bull 141:561–567
Moran AL, Woods HA (2007) Oxygen in egg masses: interactive effects of temperature, age, and egg-mass morphology on oxygen supply to embryos. J Exp Biol 210:722–731
Moreno A, Pereira J, Cunha M (2005) Environmental influences on age and size at maturity of Loligo vulgaris. Aquat Living Resour 18:377–384
Moreno A, dos Santos A, Piatkowski U, Santos AMP, Cabral H (2009) Distribution of cephalopod paralarvae in relation to the regional oceanography of the western Iberia. J Plankton Res 31:73–91
Naef A (1928) Die cephalopoden. Fauna et Flora di Golfo del Napoli Monographia 35(2):186–194
Nixon M, Mangold K (1998) The early life of Sepia officinalis, and the contract with that of Octopus vulgaris (Cephalopoda). J Zool Lond 245:407–421
O’Dor RK, Webber DM (1986) The constraints on cephalopods: why squid aren’t fish. Can J Zool 64:1591–1605
O’Dor RK, Webber DM (1991) Invertebrate athletes: trade—offs between transport efficiency and power density in cephalopod evolution. J Exp Biol 160:93–112
O’Dor RK, Wells MJ (1987) Energy and nutrient flow. In: Boyle PR (ed) Cephalopod life cycles, comparative reviews, vol 2. Academic Press, London, pp 109–133
Pechenick JA (1987) Environmental influences on larval survival and development. In: Giese AC et al (eds) Reproduction of marine invertebrates, vol IX. Blackwell Scientific Publications, New York, pp 551–608
Pechenick JA, Eyster LS, Widdows J, Bayne BL (1990) The influence of food concentration and temperature on growth and morphological differentiation of blue mussel Mytilus edulis L. larvae. J Exp Mar Biol Ecol 136:47–64
Pecl GT, Jackson GD (2008) The potential impacts of climate change on inshore squid: biology, ecology and fisheries. Rev Fish Biol Fish 18:373–385
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner HO (2002) Environmental and functional limits to muscular exercise and body size in marine invertebrates athletes. Comp Biochem Physiol A 133:303–321
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Pörtner HO, Mark FC, Bock C (2004) Oxygen limited thermal tolerance in fish? Answers obtained by nuclear magnetic resonance techniques. Resp Physiol Neurobiol 141:243–260
Pörtner HO, Langenbuch M, Michaelidis B (2005) Synergistic effects of increased CO2, temperature and hypoxia on marine animals. J Geophys Res 110:C09S10
Relvas P, Barton ED, Dubert J, Oliveira PB, Peliz A, da Silva JCB, Santos AMP (2007) Physical oceanography of the western Iberia ecosystem: latest views and challenges. Prog Oceanogr 74:149–173
Rosa R, Seibel BA (2008) Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc Natl Acad Sci USA 105:20776–20780
Rosa R, Seibel BA (2010) Metabolic physiology of the Humboldt squid, Dosidicus gigas: implications for vertical migration in a pronounced oxygen minimum zone. Progr Oceanogr 86:72–80
Rosa R, Dierssen HM, Gonzalez L, Seibel BA (2008) Large-scale diversity patterns of cephalopods in the Atlantic open ocean and deep-sea. Ecology 89:3449–3461
Rosa R, Trueblood L, Seibel BA (2009) Ecophysiological influence on scaling of aerobic and anaerobic metabolism of pelagic Gonatid squids. Physiol Biochem Zool 82:19–429
Rosa R, Pimentel MS, Boavida-Portugal J, Teixeira T, Trübenbach K, et al. (2012) Ocean warming enhances malformations, premature hatching, metabolic suppression and oxidative stress in the early life stages of a keystone squid. PLoS ONE 7(6): e38282. doi:10.1371/journal.pone.0038282
Santos FD, Forbes K, Moita R (2002) Climate change in Portugal scenarios. Impacts and adaptation measures-siam project. Gradiva, Lisbon
Sasaki GC, Capuzzo JM, Biesiot P (1986) Nutritional and bioenergetic consideration in development of the American lobster Homarus americanus. Can J Fish Aquat Sci 43:2311–2319
Seibel BA, Dymowska A, Rosenthal J (2007) Metabolic temperature compensation and coevolution of locomotory performance in pteropod molluscs. Integr Comp Biol 47:880–891
Steer MA, Moltschaniwskyj NA, Jordan AR (2003) Embryonic development of southern calamari (Sepioteuthis australis) within the constraints of an aggregated egg mass. Mar Freshw Res 54:217–226
Thorp JH, Covich AP (2001) Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego, CA, pp 315–399
Tian Y (2009) Interannual–interdecadal variations of spear squid Loligo bleekeri abundance in the southwestern Japan Sea during 1975–2006: impact of the trawl fishing and recommendations for management under the different climate regimes. Fish Res 100:78–85
Wang T, Overgaard J (2007) The heartbreak of adapting to global warming. Science 315:49–50
Ward P, Boletzky SV (1984) Shell implosion depth and implosion morphologies in three species of Sepia (Cephalopoda) from the Mediterranean Sea. J Mar Biol Assoc UK 64:955–966
Webber DM, Aitken JP, O’Dor RK (2000) Costs of locomotion and vertic dynamics of cephalopods and fish. Physiol Biochem Zool 73:651–662
Wells J, Hanlon RT, Lee PG, Dimarco FP (1988) Respiratory and cardiac performance in Lolliguncula brevis (Cephalopoda, Myopsida): the effects of activity, temperature and hypoxia. J Exp Biol 138:17–36
Wolf G, Verheyen E, Vlaeminck A, Lemaire J, Decleir W (1985) Respiration of Sepia officinalis during embryonic and early juvenile life. Mar Biol 90:35–39
Woods HA (1999) Egg-mass size and cell size: effects of temperature on oxygen distribution. Am Zool 39:244–252
Woods HA, Hill RI (2004) Temperature-dependent oxygen limitation in insect eggs. J Exp Biol 207:2267–2276
Acknowledgments
The Portuguese Foundation for Science and Technology (FCT) supported this study through project grants PTDC/BIA-BEC/103266/2008 and PTDC/MAR/0908066/2008 to R. Rosa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. O. Pörtner.
Rights and permissions
About this article
Cite this article
Pimentel, M.S., Trübenbach, K., Faleiro, F. et al. Impact of ocean warming on the early ontogeny of cephalopods: a metabolic approach. Mar Biol 159, 2051–2059 (2012). https://doi.org/10.1007/s00227-012-1991-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1991-9