Abstract
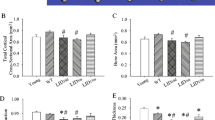
The Src homology region 2 domain-containing phosphatase-1 (SHP-1) is an intracellular tyrosine phosphatase that plays a negative regulatory role in immune cell signaling. Absent or diminished SHP-1 catalytic activity results in reduced bone mass with enhanced bone resorption. Here, we sought to investigate if Shp1 overexpression leads to increased bone mass and improved mechanical properties. Male and female wildtype (WT) and SHP1-transgenic (Tg) mice at 28, 56, and 84 days of age were compared. We applied microcomputed tomography to assess femoral cortical bone geometry and trabecular architecture and 3-point mechanical bending to assess mid-diaphyseal structural and estimated material properties. Serum OPG, RANKL, P1NP, and CTX-1 concentrations were measured by enzyme-linked immunoassay. The majority of transgene effects were restricted to the 28-day-old mice. Trabecular bone volume per total volume, trabecular number, and connectivity density were greater in 28-day-old female SHP1-Tg mice when compared to WTs. SHP1-Tg female mice showed increased total and medullary areas, with no difference in cortical area and thickness. Cortical tissue mineral density was strongly genotype-dependent. Failure load, yield load, ultimate stress, and yield stress were all lower in 28-day-old SHP1-Tg females. In 28-day-old SHP1-Tg females, circulating levels of OPG and P1NP were higher and RANKL levels were lower than WT controls. Our study demonstrates a role for SHP-1 in early postnatal bone development; SHP-1 overexpression negatively impacted whole bone strength and material properties in females.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SHP-1:
-
Src homology region 2 domain-containing phosphatase-1
- SHP1-Tg:
-
SHP-1 transgenic
- µCT:
-
Micro-computed tomography
- BV/TV:
-
Bone volume per total volume
- P1NP:
-
Procollagen type 1 N-terminal propeptide
- CTX-1:
-
C-terminal telopeptide
- OPG:
-
Osteoprotegerin
- RANKL:
-
Receptor activator of nuclear factor kappa-B ligand
- WT:
-
Wildtype
- RANK/TRAF6:
-
Receptor activator nuclear factor kappa-B/TNF receptor-associated factor 6
- NFĸb:
-
Nuclear factor kappa-B
- MSC:
-
Mesenchymal stem cell
- SIBLING:
-
Small integrin binding ligand, N-linked glycoprotein
- MEPE:
-
Matrix extracellular phosphoglycoprotein
- HRP:
-
Horseradish peroxidase
- ANOVA:
-
Analysis of variance
References
Neel BG, Tonks NK (1997) Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol 9(2):193–204
Nesterovitch AB et al (2011) Alteration in the gene encoding protein tyrosine phosphatase nonreceptor type 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. Am J Pathol 178(4):1434–1441
Nesterovitch AB et al (2011) Spontaneous insertion of a b2 element in the ptpn6 gene drives a systemic autoinflammatory disease in mice resembling neutrophilic dermatosis in humans. Am J Pathol 178(4):1701–1714
Aoki K et al (1999) The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity: increased resorption and osteopenia in me(v)/me(v) mutant mice. Bone 25(3):261–267
Granot-Attas S, Elson A (2008) Protein tyrosine phosphatases in osteoclast differentiation, adhesion, and bone resorption. Eur J Cell Biol 87(8–9):479–490
Umeda S et al (1999) Deficiency of SHP-1 protein-tyrosine phosphatase activity results in heightened osteoclast function and decreased bone density. Am J Pathol 155(1):223–233
Zhang Z, Jimi E, Bothwell AL (2003) Receptor activator of NF-kappa B ligand stimulates recruitment of SHP-1 to the complex containing TNFR-associated factor 6 that regulates osteoclastogenesis. J Immunol 171(7):3620–3626
Jiang M et al (2016) SHP1 regulates bone mass by directing mesenchymal stem cell differentiation. Cell Rep 17(8):2161
Markovics A et al (2020) Regulation of autoimmune arthritis by the SHP-1 tyrosine phosphatase. Arthritis Res Ther 22(1):160
Bouxsein ML et al (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25(7):1468–1486
Bhatia A et al (2012) Overexpression of DMP1 accelerates mineralization and alters cortical bone biomechanical properties in vivo. J Mech Behav Biomed Mater 5(1):1–8
Coggeshall KM, Nakamura K, Phee H (2002) How do inhibitory phosphatases work? Mol Immunol 39(9):521–529
Martin A et al (1999) Murine SHP-1 splice variants with altered Src homology 2 (SH2) domains. Implications for the SH2-mediated intramolecular regulation of SHP-1. J Biol Chem 274(31):21725–21734
Raab M, Rudd CE (1996) Hematopoietic cell phosphatase (HCP) regulates p56LCK phosphorylation and ZAP-70 binding to T cell receptor zeta chain. Biochem Biophys Res Commun 222(1):50–57
Chiang GG, Sefton BM (2001) Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J Biol Chem 276(25):23173–23178
Au-Yeung BB et al (2009) The structure, regulation, and function of ZAP-70. Immunol Rev 228(1):41–57
Nishizumi H et al (1998) A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals. J Exp Med 187(8):1343–1348
Xu Y et al (2005) Lyn tyrosine kinase: accentuating the positive and the negative. Immunity 22(1):9–18
Dustin LB et al (1999) Expression of dominant-negative src-homology domain 2-containing protein tyrosine phosphatase-1 results in increased Syk tyrosine kinase activity and B cell activation. J Immunol 162(5):2717–2724
Qin C, Baba O, Butler WT (2004) Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15(3):126–136
Staines KA, MacRae VE, Farquharson C (2012) The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J Endocrinol 214(3):241–255
Yang H et al (2022) Protein tyrosine phosphatases in skeletal development and diseases. Bone Res 10(1):10
Huang TT et al (2017) Alteration of SHP-1/p-STAT3 signaling: a potential target for anticancer therapy. Int J Mol Sci 18:6
Hou X, Tian F (2022) STAT3-mediated osteogenesis and osteoclastogenesis in osteoporosis. Cell Commun Signal 20(1):112
Yang Y et al (2019) STAT3 controls osteoclast differentiation and bone homeostasis by regulating NFATc1 transcription. J Biol Chem 294(42):15395–15407
Davidson RK et al (2020) The loss of STAT3 in mature osteoclasts has detrimental effects on bone structure. PLoS ONE 15(7):e0236891
Follet H et al (2004) The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone 34(5):783–789
Boskey AL et al (2010) MEPE’s diverse effects on mineralization. Calcif Tissue Int 86(1):42–46
Liu H, Xia X, Li B (2015) Mesenchymal stem cell aging: mechanisms and influences on skeletal and non-skeletal tissues. Exp Biol Med (Maywood) 240(8):1099–1106
Yuko Takeda-Matsubara HN, Iwai M, Cui TX, Shiuchi T, Masahiro Akishita CN, Ito M, Horiuchi M (2002) Estrogen activates phosphatases and antagonizes growth-promoting effect of angiotensin II. Hypertension 39:41–45
Ke K et al (2014) Reactive oxygen species induce the association of SHP-1 with c-Src and the oxidation of both to enhance osteoclast survival. Am J Physiol Endocrinol Metab 307(1):E61-70
Xu D, Rovira II, Finkel T (2002) Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev Cell 2(3):251–2
Bell MR (2018) Comparing postnatal development of gonadal hormones and associated social behaviors in rats, mice, and humans. Endocrinology 159(7):2596–2613
Acknowledgements
The authors express their gratitude to Dr. Tibor T. Glant for providing founders of the SHP1-Tg mouse strain. No funding was received to conduct this study.
Author information
Authors and Affiliations
Contributions
Study conception and design were provided by DRS, RDR, AM, and KM. Material preparation, data collection, and analysis were performed by AM, RDR, SL, and NP. The first draft of the manuscript was written by AM. Editing and critical review of previous versions of the manuscript were performed by RDR, DRS, and KM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosure
Adrienn Markovics, Sydney Lupo, Niyati Patel, Katalin Mikecz, D. Rick Sumner, and Ryan D. Ross have no relevant financial or non-financial interest to disclose.
Ethical Approval
All animal protocols were approved by the Institutional Animal Care and Use Committee (Rush University, Chicago, IL, IACUC 17–039).
Consent for Publications
All authors approve the manuscript for publication in its present form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Markovics, A., Lupo, S., Patel, N. et al. SHP-1 Protein Tyrosine Phosphatase Affects Early Postnatal Bone Development in Mice. Calcif Tissue Int 112, 472–482 (2023). https://doi.org/10.1007/s00223-023-01064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01064-5