Abstract
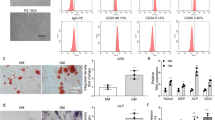
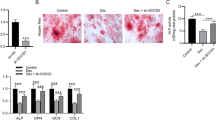
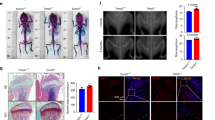
Osteoporosis is a commonly seen degenerative bone disorder in the elderly and postmenopausal women, with a low bone mineral density as a major risk factor. The osteogenic potential of bone marrow stromal cells (BMSCs) showed to be impaired during osteoporosis. We established a postmenopausal osteoporosis model in ovariectomized (OVX) mice and found the upregulation of proteasome 26S subunit ATPase 2 (PSMC2) in OVX mice. PSMC2 silencing improved OVX-impaired biomechanical properties of mice femur, OVX-decreased BMD, and OVX-destroyed bone structure. Histopathological analysis indicated that PSMC2 silencing improved bone trabecular structure and increased the contents of collagen fibers and newly formed bone or cartilage in OVX mice. In the meantime, PSMC2 silencing increased Runx2, PI3K, Wnt3a, and β-catenin protein contents while reduced CTSK protein. Within BMSCs isolated from OVX mice, PSMC2 silencing promoted BMSC osteogenic differentiation and elevated osteogenic markers’ protein contents, including HOXA10, Runx2, OCN, OPN, and COL1A2. In conclusion, PSMC2 expression is upregulated in the postmenopausal osteoporosis model in OVX mice. PSMC2 silencing promotes the osteogenic differentiation of BMSCs in vitro, promotes bone formation, and inhibits bone resorption in vivo.




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article.
References
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Kendler D (2011) Osteoporosis: therapies now and in the future. Climacteric 14:604–605
Sambrook P, Cooper C (2006) Osteoporosis Lancet 367:2010–2018
Selby P (2004) Postmenopausal osteoporosis. Curr Osteoporos Rep 2:101–106
Watts NB (1999) Postmenopausal osteoporosis. Obstet Gynecol Surv 54:532–538
Zaidi M (2007) Skeletal remodeling in health and disease. Nat Med 13:791–801
Bianco P, Sacchetti B, Riminucci M (2011) Stem cells in skeletal physiology and endocrine diseases of bone. Endocr Dev 21:91–101
Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B (2004) Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 3:379–389
Liao L, Yang X, Su X, Hu C, Zhu X, Yang N, Chen X, Shi S, Shi S, Jin Y (2013) Redundant miR-3077-5p and miR-705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow. Cell Death Dis 4:e600
Stolzing A, Scutt A (2006) Age-related impairment of mesenchymal progenitor cell function. Aging Cell 5:213–224
Liao L, Su X, Yang X, Hu C, Li B, Lv Y, Shuai Y, Jing H, Deng Z, Jin Y (2016) TNF-alpha inhibits FoxO1 by upregulating miR-705 to aggravate oxidative damage in bone marrow-derived mesenchymal stem cells during osteoporosis. Stem Cells 34:1054–1067
Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, Xia ZY, Zhou HD, Cao X, Xie H, Liao EY, Luo XH (2015) MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest 125:1509–1522
Jing H, Liao L, An Y, Su X, Liu S, Shuai Y, Zhang X, Jin Y (2016) Suppression of EZH2 prevents the shift of osteoporotic MSC fate to adipocyte and enhances bone formation during osteoporosis. Mol Ther 24:217–229
Li Y, Fan L, Hu J, Zhang L, Liao L, Liu S, Wu D, Yang P, Shen L, Chen J, Jin Y (2015) MiR-26a rescues bone regeneration deficiency of mesenchymal stem cells derived from osteoporotic mice. Mol Ther 23:1349–1357
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Zhong Z, Chen A, Fa Z, Ding Z, Xiao L, Wu G, Wang Q, Zhang R (2020) Bone marrow mesenchymal stem cells upregulate PI3K/AKT pathway and down-regulate NF-kappaB pathway by secreting glial cell-derived neurotrophic factors to regulate microglial polarization and alleviate deafferentation pain in rats. Neurobiol Dis 143:104945
Zhao SJ, Kong FQ, Jie J, Li Q, Liu H, Xu AD, Yang YQ, Jiang B, Wang DD, Zhou ZQ, Tang PY, Chen J, Wang Q, Zhou Z, Chen Q, Yin GY, Zhang HW, Fan J (2020) Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3beta/beta-catenin pathway. Theranostics 10:17–35
Todd H, Galea GL, Meakin LB, Delisser PJ, Lanyon LE, Windahl SH, Price JS (2015) Wnt16 is associated with age-related bone loss and estrogen withdrawal in murine bone. PLoS ONE 10:e0140260
Canalis E (2013) Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol 9:575–583
Rawadi G (2008) Wnt signaling and potential applications in bone diseases. Curr Drug Targets 9:581–590
Sattler AM, Schoppet M, Schaefer JR, Hofbauer LC (2004) Novel aspects on RANK ligand and osteoprotegerin in osteoporosis and vascular disease. Calcif Tissue Int 74:103–106
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Leibbrandt A, Penninger JM (2008) RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci 1143:123–150
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234
Collin-Osdoby P (1994) Role of vascular endothelial cells in bone biology. J Cell Biochem 55:304–309
Li J, Liu X, Zuo B, Zhang L (2016) The role of bone marrow microenvironment in governing the balance between osteoblastogenesis and adipogenesis. Aging Dis 7:514–525
Feng X, McDonald JM (2011) Disorders of bone remodeling. Annu Rev Pathol 6:121–145
Zhou Q, Xie F, Zhou B, Wang J, Wu B, Li L, Kang Y, Dai R, Jiang Y (2019) Differentially expressed proteins identified by TMT proteomics analysis in bone marrow microenvironment of osteoporotic patients. Osteoporos Int 30:1089–1098
Miranda M, Pino AM, Fuenzalida K, Rosen CJ, Seitz G, Rodriguez JP (2016) Characterization of fatty acid composition in bone marrow fluid from postmenopausal women: modification after hip fracture. J Cell Biochem 117:2370–2376
Pino AM, Rios S, Astudillo P, Fernandez M, Figueroa P, Seitz G, Rodriguez JP (2010) Concentration of adipogenic and proinflammatory cytokines in the bone marrow supernatant fluid of osteoporotic women. J Bone Miner Res 25:492–498
Gorzek J, Hendrickson K, Forstner J, Rixen J, Moran A, Lowe DA (2007) Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc 39:248–256
Nichterwitz S, Chen G, Aguila Benitez J, Yilmaz M, Storvall H, Cao M, Sandberg R, Deng Q, Hedlund E (2016) Laser capture microscopy coupled with Smart-seq2 for precise spatial transcriptomic profiling. Nat Commun 7:12139
Zhou B, Peng K, Wang G, Chen W, Liu P, Chen F, Kang J (2020) miR-483-3p promotes the osteogenesis of human osteoblasts by targeting Dikkopf 2 (DKK2) and the Wnt signaling pathway. Int J Mol Med 46(4):1571–1581
Qin D, Zhang H, Zhang H, Sun T, Zhao H, Lee WH (2019) Anti-osteoporosis effects of osteoking via reducing reactive oxygen species. J Ethnopharmacol 244:112045
Tao L, Shen S, Fu S, Fang H, Wang X, Das S, Sluijter JP, Rosenzweig A, Zhou Y, Kong X, Xiao J, Li X (2015) Traditional Chinese Medication Qiliqiangxin attenuates cardiac remodeling after acute myocardial infarction in mice. Sci Rep 5:8374
Martin M (2015) Docetaxel, doxorubicin and cyclophosphamide (the TAC regimen): an effective adjuvant treatment for operable breast cancer. Womens Health 2:527–537
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327:449–462
Miyazaki M, Zuk PA, Zou J, Yoon SH, Wei F, Morishita Y, Sintuu C, Wang JC (2008) Comparison of human mesenchymal stem cells derived from adipose tissue and bone marrow for ex vivo gene therapy in rat spinal fusion model. Spine (Phila Pa 1976) 33:863–869
Kameda Y, Takahata M, Mikuni S, Shimizu T, Hamano H, Angata T, Hatakeyama S, Kinjo M, Iwasaki N (2015) Siglec-15 is a potential therapeutic target for postmenopausal osteoporosis. Bone 71:217–226
Calviello G, Resci F, Serini S, Piccioni E, Toesca A, Boninsegna A, Monego G, Ranelletti FO, Palozza P (2007) Docosahexaenoic acid induces proteasome-dependent degradation of beta-catenin, down-regulation of survivin and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinogenesis 28:1202–1209
Kimelman D, Xu W (2006) beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25:7482–7491
Takada I, Kouzmenko AP, Kato S (2009) Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 5:442–447
Nishikawa K, Nakashima T, Takeda S, Isogai M, Hamada M, Kimura A, Kodama T, Yamaguchi A, Owen MJ, Takahashi S, Takayanagi H (2010) Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest 120:3455–3465
Yang K, Chen Z, Gao J, Shi W, Li L, Jiang S, Hu H, Liu Z, Xu D, Wu L (2017) The key roles of GSK-3beta in regulating mitochondrial activity. Cell Physiol Biochem 44:1445–1459
Hur EM, Zhou FQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11:539–551
Zhao R (2012) Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci 9:825–832
Panwar P, Xue L, Soe K, Srivastava K, Law S, Delaisse JM, Bromme D (2017) An ectosteric inhibitor of cathepsin K inhibits bone resorption in ovariectomized mice. J Bone Miner Res 32:2415–2430
Liu YQ, Hong ZL, Zhan LB, Chu HY, Zhang XZ, Li GH (2016) Wedelolactone enhances osteoblastogenesis by regulating Wnt/beta-catenin signaling pathway but suppresses osteoclastogenesis by NF-kappaB/c-fos/NFATc1 pathway. Sci Rep 6:32260
Bidwell JP, Alvarez MB, Hood M Jr, Childress P (2013) Functional impairment of bone formation in the pathogenesis of osteoporosis: the bone marrow regenerative competence. Curr Osteoporos Rep 11:117–125
Funding
This study was supported by Innovative Research and Development project of Hunan Development and Reform Commission (2019412).
Author information
Authors and Affiliations
Contributions
Bin Zhou performed the experiments and wrote the paper. Kun Peng and Weihua Chen coordinated the study. Guoqiang Wang collected the samples and assisted with the experiments. Yijun Kang conceived the study, designed the experiments, and revised the paper. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosure
Bin Zhou, Kun Peng, Guoqiang Wang, Weihua Chen, and Yijun Kang declared that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All experiments were carried out according to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and were approved by the Ethics Committee of the Second Xiangya Hospital, Central South University. There are no human sujects in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, B., Peng, K., Wang, G. et al. Silencing Proteasome 26S Subunit ATPase 2 (PSMC2) Protects the Osteogenic Differentiation In Vitro and Osteogenesis In Vivo. Calcif Tissue Int 109, 44–54 (2021). https://doi.org/10.1007/s00223-021-00819-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00819-2