Abstract
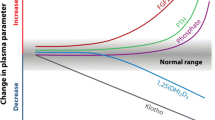
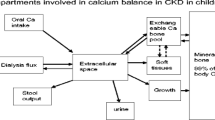
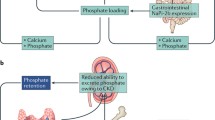
Hyperphosphatemia is common in chronic kidney disease (CKD). Often seen as the “silent killer” because of its dramatic effect on vascular calcifications, hyperphosphatemia explains, at least partly, the onset of the complex mineral and bone disorders associated with CKD (CKD–MBD), together with hypocalcemia and decreased 1-25(OH)2 vitamin D levels. The impact of CKD–MBD may be immediate with abnormalities of bone and mineral metabolism with secondary hyperparathyroidism and increased FGF23 levels, or delayed with poor growth, bone deformities, fractures, and vascular calcifications, leading to increased morbidity and mortality. The global management of CKD–MBD has been detailed in international guidelines for adults and children, however, with difficulties to obtain an agreement on the ideal PTH targets. The clinical management of hyperphosphatemia is a daily challenge for nephrologists and pediatric nephrologists, notably because of the phosphate overload in occidental diets that is mainly due to the phosphate “hidden” in food additives. The management begins with a dietary restriction of phosphate intake, and is followed by the use of calcium-based and non-calcium-based phosphate binders, and/or the intensification of dialysis. The objective of this review is to provide an overview of the pathophysiology of hyperphosphatemia in CKD, with a focus on its deleterious effects and a description of the clinical management of hyperphosphatemia in a more global setting of CKD–MBD.

Similar content being viewed by others
References
Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23(4):578–585
Shroff R (2013) Phosphate is a vascular toxin. Pediatr Nephrol 28(4):583–593
Shroff R, Long DA, Shanahan C (2013) Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24(2):179–189
Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K et al (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 69(11):1945–1953
Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D et al (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 342(20):1478–1483
Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U et al (2002) Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106(1):100–105
Cejka D, Weber M, Diarra D, Reiter T, Kainberger F, Haas M (2014) Inverse association between bone microarchitecture assessed by HR-pQCT and coronary artery calcification in patients with end-stage renal disease. Bone 64:33–38
Malluche HH, Blomquist G, Monier-Faugere M-C, Cantor TL, Davenport DL (2015) High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol 26(10):2534–2544
Ziolkowska H, Brzewski M, Roszkowska-Blaim M (2008) Determinants of the intima-media thickness in children and adolescents with chronic kidney disease. Pediatr Nephrol. 23(5):805–811
Preka E, Ranchin B, Doyon A, Vierge M, Ginhoux T, Kassai B et al (2018) The interplay between bone and vessels in pediatric CKD: lessons from a single-center study. Pediatr Nephrol. 33:1565–1575
Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD–MBD). Kidney Int Supl 113:S1–130
Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L et al (2017) Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD–MBD) guideline update: what’s changed and why it matters. Kidney Int 92(1):26–36
Shroff R, Wan M, Nagler EV, Bakkaloglu S, Cozzolino M, Bacchetta J et al (2017) Clinical practice recommendations for treatment with active vitamin D analogues in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol Dial Transplant 32(7):1114–1127
Klaus G, Watson A, Edefonti A, Fischbach M, Rönnholm K, Schaefer F et al (2006) Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatr Nephrol 21(2):151–159
Drube J, Wan M, Bonthuis M, Wühl E, Bacchetta J, Santos F et al (2019) Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat Rev Nephrol 15(9):577–589
Ritz E, Hahn K, Ketteler M, Kuhlmann MK, Mann J (2012) Phosphate additives in food–a health risk. Dtsch Arztebl Int 109(4):49–55
Calvo MS, Uribarri J (2013) Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr 98(1):6–15
ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26(3):345–348
Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H et al (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79(12):1370–1378
Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A et al (2008) Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359(6):584–592
Kurosu H, Kuro-O M (2009) The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol 299(1):72–78
Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H et al (2008) Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res 23(9):1509–1518
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T et al (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113(4):561–568
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M et al (2007) The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117(12):4003–4008
Portale AA, Wolf MS, Messinger S, Perwad F, Jüppner H, Warady BA et al (2016) Fibroblast growth factor 23 and risk of CKD progression in children. Clin J Am Soc Nephrol 11(11):1989–1998
Wesseling-Perry K, Pereira RC, Tseng C-H, Elashoff R, Zaritsky JJ, Yadin O et al (2012) Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 7(1):146–152
Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K et al (2010) Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 77(3):232–238
Bacchetta J, Bardet C, Prié D (2019) Physiology of FGF23 and overview of genetic diseases associated with renal phosphate wasting. Metab Clin Exp. https://doi.org/10.1016/j.metabol.2019.01.006
Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T et al (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121(11):4393–4408
Olauson H, Lindberg K, Amin R, Sato T, Jia T, Goetz R et al (2013) Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet 9(12):e1003975
Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B et al (2013) Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res 28(1):46–55
Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK (2016) Low Vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 27(1):227–237
Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B et al (2014) FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6(6):744–759
Hensel N, Schön A, Konen T, Lübben V, Förthmann B, Baron O et al (2016) Fibroblast growth factor 23 signaling in hippocampal cells: impact on neuronal morphology and synaptic density. J Neurochem 137(5):756–769
Gardner J, Ashraf A, You Z, McCormick K (2011) Changes in plasma FGF23 in growth hormone deficient children during rhGH therapy. J Pediatr Endocrinol Metab 24(9–10):645–650
Rutkowski JM, Pastor J, Sun K, Park SK, Bobulescu IA, Chen CT et al (2017) Adiponectin alters renal calcium and phosphate excretion through regulation of klotho expression. Kidney Int 91(2):324–337
Denburg MR, Kalkwarf HJ, de Boer IH, Hewison M, Shults J, Zemel BS et al (2013) Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatr Nephrol 28(9):1843–1853
Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S et al (2016) Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90(5):985–996
Eisenga MF, van Londen M, Leaf DE, Nolte IM, Navis G, Bakker SJL et al (2017) C-Terminal fibroblast growth factor 23, iron deficiency, and mortality in renal transplant recipients. J Am Soc Nephrol 28(12):3639–3646
Wolf M, Koch TA, Bregman DB (2013) Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28(8):1793–1803
Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ (2006) 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int 70(4):654–659
Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C et al (2012) Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clin J Am Soc Nephrol 7(2):216–223
Westerberg P-A, Sterner G, Ljunggren Ö, Isaksson E, Elvarson F, Dezfoolian H et al (2017) High doses of cholecalciferol alleviate the progression of hyperparathyroidism in patients with CKD Stages 3–4: results of a 12-week double-blind, randomized, controlled study. Nephrol Dial Transplant 33:466–471
Snauwaert E, Van Biesen W, Raes A, Glorieux G, Vanholder R, Vande Walle J et al (2018) A plea for more uremic toxin research in children with chronic kidney disease. Pediatr Nephrol 33(6):921–924
Kuro-o M (2010) A potential link between phosphate and aging–lessons from Klotho-deficient mice. Mech Ageing Dev 131(4):270–275
Gutiérrez OM, Anderson C, Isakova T, Scialla J, Negrea L, Anderson AH et al (2010) Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol 21(11):1953–1960
Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang H-Y, Crouthamel MH et al (2013) Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 83(6):1159–1168
Ardeshirpour L, Cole DEC, Carpenter TO (2007) Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev 5(Suppl 1):584–598
KDOQI Work Group (2009) KDOQI Clinical practice guideline for nutrition in children with CKD: 2008 update executive summary. Am J Kidney Dis 53(3 Suppl 2):S11–104
Fouque D, Roth H, Pelletier S, London GM, Hannedouche T, Jean G et al (2013) Control of mineral metabolism and bone disease in haemodialysis patients: which optimal targets? Nephrol Dial Transplant 28(2):360–367
Rinat C, Becker-Cohen R, Nir A, Feinstein S, Shemesh D, Algur N et al (2010) A comprehensive study of cardiovascular risk factors, cardiac function and vascular disease in children with chronic renal failure. Nephrol Dial Transplant 25(3):785–793
Mizobuchi M, Finch JL, Martin DR, Slatopolsky E (2007) Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int 72(6):709–715
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM (2004) Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15(8):2208–2218
Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA (2009) Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20(2):397–404
Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Gaziano JM et al (2007) Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167(9):879–885
Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D et al (2007) Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18(11):2996–3003
Mannstadt M, Bilezikian JP, Thakker RV, Hannan FM, Clarke BL, Rejnmark L et al (2017) Hypoparathyroidism. Nat Rev Dis Primers 3:17055
Mantovani G, Bastepe M, Monk D, de Sanctis L, Thiele S, Usardi A et al (2018) Diagnosis and management of pseudohypoparathyroidism and related disorders: first international Consensus Statement. Nat Rev Endocrinol 14(8):476–500
Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M et al (2004) Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36(6):579–581
Claramunt-Taberner D, Bertholet-Thomas A, Carlier M-C, Dijoud F, Chotel F, Silve C et al (2018) Hyperphosphatemic tumoral calcinosis caused by FGF23 compound heterozygous mutations: what are the therapeutic options for a better control of phosphatemia? Pediatr Nephrol 33(7):1263–1267
Chang AR, Lazo M, Appel LJ, Gutiérrez OM, Grams ME (2014) High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr 99(2):320–327
Weinman EJ, Light PD, Suki WN (2013) Gastrointestinal phosphate handling in CKD and its association with cardiovascular disease. Am J Kidney Dis 62(5):1006–1011
Khairallah P, Isakova T, Asplin J, Hamm L, Dobre M, Rahman M et al (2017) Acid Load and Phosphorus Homeostasis in CKD. Am J Kidney Dis 70(4):541–550
Shroff R, Wan M, Nagler EV, Bakkaloglu S, Fischer D-C, Bishop N et al (2017) Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol Dial Transplant 32(7):1098–1113
Bacchetta J, Schmitt CP, Ariceta G, Bakkaloglu SA, Groothoff J, Wan M et al (2019) Cinacalcet use in paediatric dialysis: a position statement from the European Society for Paediatric Nephrology and the Chronic Kidney Disease-Mineral and Bone Disorders Working Group of the ERA-EDTA. Nephrol Dial Transplant 35(1):47–64
Denburg MR, Tsampalieros AK, de Boer IH, Shults J, Kalkwarf HJ, Zemel BS et al (2013) Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J Clin Endocrinol Metab 98(5):1930–1938
Bakkaloglu SA, Wesseling-Perry K, Pereira RC, Gales B, Wang H-J, Elashoff RM et al (2010) Value of the new bone classification system in pediatric renal osteodystrophy. Clin J Am Soc Nephrol 5(10):1860–1866
Denburg MR, Kumar J, Jemielita T, Brooks ER, Skversky A, Portale AA et al (2016) Fracture Burden and Risk Factors in Childhood CKD: Results from the CKiD Cohort Study. J Am Soc Nephrol 27(2):543–550
Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R (2000) Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res 15(11):2245–2250
Hahn D, Hodson EM, Craig JC (2015) Interventions for metabolic bone disease in children with chronic kidney disease. Cochrane Database Syst Rev 11:CD008327
Sherman RA, Ravella S, Kapoian T (2015) The phosphate content of prescription medication: a new consideration. Ther Innov Regul Sci 49(6):886–889
Hannedouche T, Fouque D, Joly D (2018) Metabolic complications in chronic kidney disease: hyperphosphatemia, hyperkalemia and anemia. Nephrol Ther 14(6):6S17–16S25
Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL et al (2011) Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6(2):257–264
Scialla JJ, Appel LJ, Wolf M, Yang W, Zhang X, Sozio SM et al (2012) Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: the chronic renal insufficiency cohort study. J Ren Nutr 22(4):379–388.e1
Sullivan C, Sayre SS, Leon JB, Machekano R, Love TE, Porter D et al (2009) Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA 301(6):629–635
Liu Z, Su G, Guo X, Wu Y, Liu X, Zou C et al (2015) Dietary interventions for mineral and bone disorder in people with chronic kidney disease. Cochrane Database Syst Rev 9:CD010350
Sullivan CM, Leon JB, Sehgal AR (2007) Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr 17(5):350–354
Lindley E, Costelloe S, Bosomworth M, Fouque D, Freeman J, Keane D et al (2014) Use of a standard urine assay for measuring the phosphate content of beverages. J Ren Nutr 24(6):353–356
D’Alessandro C, Piccoli GB, Cupisti A (2015) The, “phosphorus pyramid”: a visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol 16:9
Isakova T, Gutiérrez OM, Chang Y, Shah A, Tamez H, Smith K et al (2009) Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20(2):388–396
Gonzalez E, Schomberg J, Amin N, Salusky IB, Zaritsky J (2010) Sevelamer carbonate increases serum bicarbonate in pediatric dialysis patients. Pediatr Nephrol 25(2):373–375
Jin C, Gao L, Li Y, Wu S, Lu X, Yang J et al (2017) Lanthanum damages learning and memory and suppresses astrocyte-neuron lactate shuttle in rat hippocampus. Exp Brain Res 235(12):3817–3832
Floege J, Covic AC, Ketteler M, Mann JFE, Rastogi A, Spinowitz B et al (2015) Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 30(6):1037–1046
Hanudel MR, Laster M, Ramos G, Gales B, Salusky IB (2018) Clinical experience with the use of ferric citrate as a phosphate binder in pediatric dialysis patients. Pediatr Nephrol 33(11):2137–2142
Kalantar-Zadeh K, Parameswaran V, Ficociello LH, Anderson L, Ofsthun NJ, Kwoh C et al (2018) Real-world scenario improvements in serum phosphorus levels and pill burden in peritoneal dialysis patients treated with sucroferric oxyhydroxide. Am J Nephrol 47(3):153–161
Ix JH, Isakova T, Larive B, Raphael KL, Raj DS, Cheung AK et al (2019) Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: the COMBINE Trial. J Am Soc Nephrol 30(6):1096–1108
Floege J (2019) Phosphate binders in chronic kidney disease: an updated narrative review of recent data. J Nephrol. https://doi.org/10.1007/s40620-019-00689-w
Kaesler N, Goettsch C, Weis D, Schurgers L, Hellmann B, Floege J et al (2019) Magnesium but not nicotinamide prevents vascular calcification in experimental uraemia. Nephrol Dial Transplant 35(1):65–73
Daugirdas JT, Finn WF, Emmett M, Chertow GM, Frequent Hemodialysis Network Trial Group (2011) The phosphate binder equivalent dose. Semin Dial 24(1):41–49
Pereira RC, Jüppner H, Gales B, Salusky IB, Wesseling-Perry K (2015) Osteocytic protein expression response to doxercalciferol therapy in pediatric dialysis patients. PLoS ONE 10(3):e0120856
Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R et al (2015) Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 132(1):27–39
Oliveira RB, Cancela ALE, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L et al (2010) Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD–MBD therapy? Clin J Am Soc Nephrol 5(2):286–291
Liabeuf S, Ryckelynck J-P, El Esper N, Ureña P, Combe C, Dussol B et al (2017) Randomized clinical trial of sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin J Am Soc Nephrol 12(12):1930–1940
Iguchi A, Kazama JJ, Yamamoto S, Yoshita K, Watanabe Y, Iino N et al (2015) Administration of ferric citrate hydrate decreases circulating FGF23 levels independently of serum phosphate levels in hemodialysis patients with iron deficiency. Nephron 131(3):161–166
Ketteler M, Sprague SM, Covic AC, Rastogi A, Spinowitz B, Rakov V et al (2019) Effects of sucroferric oxyhydroxide and sevelamer carbonate on chronic kidney disease-mineral bone disorder parameters in dialysis patients. Nephrol Dial Transplant 34(7):1163–1170
Cochat P, Rumsby G (2013) Primary hyperoxaluria. N Engl J Med 369(7):649–658
Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP et al (2008) Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 88(6):1511–1518
Bacchetta J (2019) Treatment of hyperphosphatemia: the dangers of high PTH levels. Pediatr Nephrol 5:493–500
Pelletier S, Fouque D (2011) Mineral and bone metabolism in dialysis: towards unified patient care? Nephrol Dial Transplant 26(1):7–9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Justine Bacchetta, Julie Bernardor, Charlotte Garnier, Corentin Naud, and Bruno Ranchin declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bacchetta, J., Bernardor, J., Garnier, C. et al. Hyperphosphatemia and Chronic Kidney Disease: A Major Daily Concern Both in Adults and in Children. Calcif Tissue Int 108, 116–127 (2021). https://doi.org/10.1007/s00223-020-00665-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00665-8