Abstract
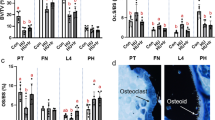
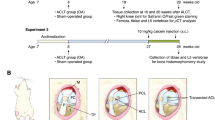
The mechano-adaptive response of bone to loading in the murine uniaxial tibial loading model is impaired in aged animals. Previous studies have shown that in aged mice, the amount of bone formed in response to loading is augmented when loads are applied following sciatic neurectomy. The synergistic effect of neurectomy and loading remains to be elucidated. We hypothesize that sciatic neurectomy increases cellular presence, thereby augmenting the response to load in aged mice. We examined bone adaptation in four groups of female C57BL/6J mice, 20–22 months old: (1) sham surgery + 9N loading; (2) sciatic neurectomy, sacrificed after 5 days; (3) sciatic neurectomy, sacrificed after 19 days; (4) sciatic neurectomy + 9N loading. We examined changes in bone cross sectional properties with micro-CT images, and static and dynamic histomorphometry with histological sections taken at the midpoint between tibiofibular junctions. The response to loading at 9N was not detectable with quantitative micro-CT data, but surface-specific histomorphometry captured an increase in bone formation in specific regions. 5 days following sciatic neurectomy, the amount of bone in the neurectomized leg was the same as the contralateral leg, but 19 days following sciatic neurectomy, there was significant bone loss in the neurectomized leg, and both osteoclasts and osteoblasts were recruited to the endosteal surfaces. When sciatic neurectomy and loading at 9N were combined, 3 out of 4 bone quadrants had increased bone formation, on the endosteal and periosteal surfaces (increased osteoid surface and mineralizing surface respectively). These data demonstrate that sciatic neurectomy increases cellular presence on the endosteal surface. With long-term sciatic-neurectomy, both osteoclasts and osteoblasts were recruited to the endosteal surface, which resulted in increased bone formation when combined with a sufficient mechanical stimulus. Controlled and localized recruitment of both osteoblasts and osteoclasts combined with appropriate mechanical loading could inform therapies for mechanically-directed bone formation.







Similar content being viewed by others
References
Fritton J, Myers E, Wright T, Vandermeulen M (2005) Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone 36:1030–1038. https://doi.org/10.1016/j.bone.2005.02.013
Willie BM, Birkhold AI, Razi H, Thiele T, Aido M, Kruck B et al (2013) Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone 55:335–346. https://doi.org/10.1016/j.bone.2013.04.023
Yang H, Butz KD, Duffy D, Niebur GL, Nauman EA, Main RP (2014) Characterization of cancellous and cortical bone strain in the in vivo mouse tibial loading model using microCT-based finite element analysis. Bone 66:131–139. https://doi.org/10.1016/j.bone.2014.05.019
Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS (2011) Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone 49:184–193. https://doi.org/10.1016/j.bone.2011.03.683
De Souza RL, Matsuura M, Eckstein F, Rawlinson SCF, Lanyon LE, Pitsillides AA (2005) Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37:810–818. https://doi.org/10.1016/j.bone.2005.07.022
Weatherholt AM, Fuchs RK, Warden SJ (2013) Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone 52:372–379. https://doi.org/10.1016/j.bone.2012.10.026
Sun D, Brodt MD, Zannit HM, Holguin N, Silva MJ (2017) Evaluation of loading parameters for murine axial tibial loading: stimulating cortical bone formation while reducing loading duration. J Orthop Res. https://doi.org/10.1002/jor.23727
McKenzie JA, Bixby EC, Silva MJ (2011) Differential gene expression from microarray analysis distinguishes woven and lamellar bone formation in the rat ulna following mechanical loading. PLoS ONE. 6:e29328. https://doi.org/10.1371/journal.pone.0029328
McBride SH, Silva MJ (2012) Adaptive and injury response of bone to mechanical loading. BoneKEy Rep 1:192. https://doi.org/10.1038/bonekey.2012.192
Holguin N, Brodt MD, Sanchez ME, Silva MJ (2014) Aging diminishes lamellar and woven bone formation induced by tibial compression in adult C57BL/6. Bone 65:83–91. https://doi.org/10.1016/j.bone.2014.05.006
Birkhold AI, Razi H, Duda GN, Weinkamer R, Checa S, Willie BM (2014) The influence of age on adaptive bone formation and bone resorption. Biomaterials 35:9290–9301. https://doi.org/10.1016/j.biomaterials.2014.07.051
Srinivasan S (2003) Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone. https://doi.org/10.1016/s8756-3282(03)00274-6
Razi H, Birkhold AI, Weinkamer R, Duda GN, Willie BM, Checa S (2015) Aging leads to a dysregulation in mechanically driven bone formation and resorption. J Bone Miner Res 30:1864–1873. https://doi.org/10.1002/jbmr.2528
Meakin LB, Galea GL, Sugiyama T, Lanyon LE, Price JS (2014) Age-related impairment of bones’ adaptive response to loading in mice is associated with sex-related deficiencies in osteoblasts but no change in osteocytes. J Bone Miner Res 29:1859–1871. https://doi.org/10.1002/jbmr.2222
Marie PJ (2014) Bone cell senescence: mechanisms and perspectives. J Bone Miner Res 29:1311–1321. https://doi.org/10.1002/jbmr.2190
Almeida M, O’Brien CA (2013) Basic biology of skeletal aging: role of stress response pathways. J Gerontol Ser A 68:1197–1208. https://doi.org/10.1093/gerona/glt079
Courtland H-W, Kennedy OD, Wu Y, Gao Y, Sun H, Schaffler MB et al (2013) Low levels of plasma IGF-1 inhibit intracortical bone remodeling during aging. Age (Dordr). 35:1691–1703. https://doi.org/10.1007/s11357-012-9469-8
Gong Z, Kennedy O, Sun H, Wu Y, Williams GA, Klein L et al (2014) Reductions in serum IGF-1 during aging impair health span. Aging Cell 13:408–418. https://doi.org/10.1111/acel.12188
Riggs BL, Khosla S, Melton LJ (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302. https://doi.org/10.1210/edrv.23.3.0465
Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O’Brien CA et al (2010) Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 9:147–161. https://doi.org/10.1111/j.1474-9726.2009.00545.x
Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS et al (2010) Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration: Antioxidant effect of anabolic intermittent PTH therapy. Aging Cell 9:851–867. https://doi.org/10.1111/j.1474-9726.2010.00616.x
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK et al (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297. https://doi.org/10.1074/jbc.M702810200
Ambrogini E, Almeida M, Martin-Millan M, Paik J-H, DePinho RA, Han L et al (2010) FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab 11:136–146. https://doi.org/10.1016/j.cmet.2009.12.009
Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L et al (2013) Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res 28:2414–2430. https://doi.org/10.1002/jbmr.1971
Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE (1999) Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab 17:171–177
Muschler GF, Nitto H, Boehm CA, Easley KA (2001) Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res 19:117–125. https://doi.org/10.1016/S0736-0266(00)00010-3
D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA (1999) Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14:1115–1122. https://doi.org/10.1359/jbmr.1999.14.7.1115
Kahn A, Gibbons R, Perkins S, Gazit D (1995) Age-related bone loss. A hypothesis and initial assessment in mice. Clin Orthop Relat Res 313:69–75
Yang Y-M, Li P, Cui D-C, Dang R-J, Zhang L, Wen N et al (2015) Effect of aged bone marrow microenvironment on mesenchymal stem cell migration. Age. https://doi.org/10.1007/s11357-014-9743-z
Koshihara Y, Suematsu A, Feng D, Okawara R, Ishibashi H, Yamamoto S (2002) Osteoclastogenic potential of bone marrow cells increases with age in elderly women with fracture. Mech Ageing Dev 123:1321–1331. https://doi.org/10.1016/S0047-6374(02)00071-4
Perkins SL, Gibbons R, Kling S, Kahn AJ (1994) Age-related bone loss in mice is associated with an increased osteoclast progenitor pool. Bone 15:65–72. https://doi.org/10.1016/8756-3282(94)90893-1
Cao JJ, Wronski TJ, Iwaniec U, Phleger L, Kurimoto P, Boudignon B et al (2005) Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J Bone Miner Res 20:1659–1668. https://doi.org/10.1359/JBMR.050503
Holguin N, Brodt MD, Silva MJ (2016) Activation of Wnt signaling by mechanical loading is impaired in the bone of old mice. J Bone Miner Res 31:2215–2226. https://doi.org/10.1002/jbmr.2900
Galea GL, Meakin LB, Harris MA, Delisser PJ, Lanyon LE, Harris SE et al (2017) Old age and the associated impairment of bones’ adaptation to loading are associated with transcriptomic changes in cellular metabolism, cell–matrix interactions and the cell cycle. Gene 599:36–52. https://doi.org/10.1016/j.gene.2016.11.006
Sakai A, Mori T, Sakuma-Zenke M, Takeda T, Nakai K, Katae Y et al (2005) Osteoclast development in immobilized bone is suppressed by parathyroidectomy in mice. J Bone Miner Metab 23:8–14. https://doi.org/10.1007/s00774-004-0534-y
Weinreb M, Rodan GA, Thompson DD (1989) Osteopenia in the immobilized rat hind limb is associated with increased bone resorption and decreased bone formation. Bone 10:187–194. https://doi.org/10.1016/8756-3282(89)90052-5
Meakin LB, Delisser PJ, Galea GL, Lanyon LE, Price JS (2015) Disuse rescues the age-impaired adaptive response to external loading in mice. Osteoporos Int 26:2703–2708. https://doi.org/10.1007/s00198-015-3142-x
Souza RD, Javaheri B, Collinson RS, Chenu C, Shefelbine SJ, Lee PD et al (2017) Prolonging disuse in aged mice amplifies cortical but not trabecular bones’ response to mechanical loading. J Musculoskelet Neuronal Interact 17:218–225
Sugawara Y, Kamioka H, Ishihara Y, Fujisawa N, Kawanabe N, Yamashiro T (2013) The early mouse 3D osteocyte network in the presence and absence of mechanical loading. Bone 52:189–196. https://doi.org/10.1016/j.bone.2012.09.033
Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE et al (2012) Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int 23:1225–1234. https://doi.org/10.1007/s00198-011-1656-4
Batt JAE, Bain JR (2013) Tibial nerve transection—a standardized model for denervation-induced skeletal muscle atrophy in mice. JoVE. https://doi.org/10.3791/50657
Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS et al (2010) BoneJ: free and extensible bone image analysis in ImageJ. Bone 47:1076–1079. https://doi.org/10.1016/j.bone.2010.08.023
Pereira AF, Javaheri B, Pitsillides AA, Shefelbine SJ (2015) Predicting cortical bone adaptation to axial loading in the mouse tibia. J R Soc Interface 12:20150590. https://doi.org/10.1098/rsif.2015.0590
Ferro Pereira A (2014) Cortical bone adaptation: a finite-element study of the mouse tibia. http://spiral.imperial.ac.uk/handle/10044/1/24716
Carriero A, Pereira AF, Wilson AJ, Castagno S, Javaheri B, Pitsillides AA et al (2018) Spatial relationship between bone formation and mechanical stimulus within cortical bone: Combining 3D fluorochrome mapping and poroelastic finite element modelling. Bone Rep 8:72–80. https://doi.org/10.1016/j.bonr.2018.02.003
Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS et al (2008) Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone 43:238–248. https://doi.org/10.1016/j.bone.2008.04.012
Sugiyama T, Price JS, Lanyon LE (2010) Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone 46:314–321. https://doi.org/10.1016/j.bone.2009.08.054
Javaheri B, Carriero A, Wood M, De Souza R, Lee PD, Shefelbine S et al (2018) Transient peak-strain matching partially recovers the age-impaired mechanoadaptive cortical bone response. Sci Rep. https://doi.org/10.1038/s41598-018-25084-6
Piet J, Hu D, Baron R, Shefelbine SJ (2019) Bone adaptation compensates resorption when sciatic neurectomy is followed by low magnitude induced loading. Bone 120:487–494. https://doi.org/10.1016/j.bone.2018.12.017
Pataky TC (2012) One-dimensional statistical parametric mapping in Python. Comput Methods Biomech Biomed Eng 15:295–301. https://doi.org/10.1080/10255842.2010.527837
Pataky TC (2010) Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J Biomech 43:1976–1982. https://doi.org/10.1016/j.jbiomech.2010.03.008
Burt-Pichat B, Lafage-Proust MH, Duboeuf F, Laroche N, Itzstein C, Vico L et al (2005) Dramatic decrease of innervation density in bone after ovariectomy. Endocrinology 146:503–510. https://doi.org/10.1210/en.2004-0884
Schmalbruch H (1986) Fiber composition of the rat sciatic nerve. Anat Rec 215:71–81. https://doi.org/10.1002/ar.1092150111
Elefteriou F, Campbell P, Ma Y (2014) Control of bone remodeling by the peripheral sympathetic nervous system. Calcif Tissue Int 94:140–151. https://doi.org/10.1007/s00223-013-9752-4
Kondo H, Nifuji A, Takeda S, Ezura Y, Rittling SR, Denhardt DT et al (2005) Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J Biol Chem 280:30192–30200. https://doi.org/10.1074/jbc.M504179200
Sseur R, Sabatier J-P, Potrel-Burgot C, Lecoq B, Creveuil C, Marcelli C (2003) Sympathetic nervous system as transmitter of mechanical loading in bone. Joint Bone Spine. 70:515–519
de Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C (2005) Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res 20:2159–2168. https://doi.org/10.1359/JBMR.050812
Marenzana M, De Souza RL, Chenu C (2007) Blockade of beta-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone 41:206–215. https://doi.org/10.1016/j.bone.2007.04.184
Meakin LB, Todd H, Delisser PJ, Galea GL, Moustafa A, Lanyon LE et al (2017) Parathyroid hormone’s enhancement of bones’ osteogenic response to loading is affected by ageing in a dose- and time-dependent manner. Bone 98:59–67. https://doi.org/10.1016/j.bone.2017.02.009
Pountos I, Giannoudis PV, Jones E, English A, Churchman S, Field S et al (2011) NSAIDS inhibit in vitro MSC chondrogenesis but not osteogenesis: implications for mechanism of bone formation inhibition in man. J Cell Mol Med 15:525–534. https://doi.org/10.1111/j.1582-4934.2010.01006.x
Oh N, Kim S, Hosoya K, Okumura M (2014) Compensatory cellular reactions to nonsteroidal anti-inflammatory drugs on osteogenic differentiation in canine bone marrow-derived mesenchymal stem cells. J Vet Med Sci 76:629–636. https://doi.org/10.1292/jvms.13-0482
Hoggatt J, Mohammad KS, Singh P, Hoggatt AF, Chitteti BR, Speth JM et al (2013) Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature 495:365–369. https://doi.org/10.1038/nature11929
García-Martínez O, De Luna-Bertos E, Ramos-Torrecillas J, Manzano-Moreno FJ, Ruiz C (2015) Repercussions of NSAIDS drugs on bone tissue: the osteoblast. Life Sci 123:72–77. https://doi.org/10.1016/j.lfs.2015.01.009
Funding
This work received internal funding from Northeastern University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All animal procedures received approval from Northeastern University’s Institutional Animal Care and Use Committee (IACUC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Piet, J., Hu, D., Meslier, Q. et al. Increased Cellular Presence After Sciatic Neurectomy Improves the Bone Mechano-adaptive Response in Aged Mice. Calcif Tissue Int 105, 316–330 (2019). https://doi.org/10.1007/s00223-019-00572-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00572-7