Abstract
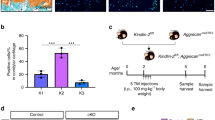
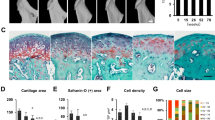
Osteoprotegerin (OPG) is one of the protective factors of bony tissue. However, the function of OPG in cartilage tissues remains elusive. The aim of this study is to explore the function of OPG in the postnatal maintenance and the occurring of osteoarthritis (OA) of temporomandibular joint (TMJ) in the rodent models. We found that OPG expressed in the hypertrophic layer of the condylar cartilage and upregulated in the hyperocclusion-induced-TMJ-trauma rat. In the absence of OPG, the cartilage degradation occurred prior to that in WT mice, and the 3-month-old OPG-Knockout (OPG-KO) condyle showed decreased chondrocyte proliferation and increased chondrocyte apoptosis, whereas the number of chondroclasts was comparable to WT condyle. The isolated chondrocytes from the OPG-KO mice also showed impaired survival and promoted chondrogenic differentiation. Furthermore, the hyperocclusion model deteriorated TMJ degradation in the OPG-KO mice. OPG plays a protective role in the condylar chondrocytes’ survival, and it is required for the postnatal maintenance of TMJ.







Similar content being viewed by others
References
Liu F, Steinkeler A (2013) Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent Clin North Am 57(3):465–479. https://doi.org/10.1016/j.cden.2013.04.006
Wang XD, Zhang JN, Gan YH, Zhou YH (2015) Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res 94(5):666–673. https://doi.org/10.1177/0022034515574770
Schmitter M, Essig M, Seneadza V, Balke Z, Schroder J, Rammelsberg P (2010) Prevalence of clinical and radiographic signs of osteoarthrosis of the temporomandibular joint in an older persons community. Dentomaxillofac Radiol 39(4):231–234. https://doi.org/10.1259/dmfr/16270943
Zhao YP, Zhang ZY, Wu YT, Zhang WL, Ma XC (2011) Investigation of the clinical and radiographic features of osteoarthrosis of the temporomandibular joints in adolescents and young adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111(2):e27–e34. https://doi.org/10.1016/j.tripleo.2010.09.076
Bellido M, Lugo L, Roman-Blas JA, Castaneda S, Caeiro JR, Dapia S, Calvo E, Largo R, Herrero-Beaumont G (2010) Subchondral bone microstructural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res Ther 12(4):R152. https://doi.org/10.1186/ar3103
Tat SK, Pelletier JP, Lajeunesse D, Fahmi H, Duval N, Martel-Pelletier J (2008) Differential modulation of RANKL isoforms by human osteoarthritic subchondral bone osteoblasts: influence of osteotropic factors. Bone 43(2):284–291. https://doi.org/10.1016/j.bone.2008.04.006
Massicotte F, Lajeunesse D, Benderdour M, Pelletier JP, Hilal G, Duval N, Martel-Pelletier J (2002) Can altered production of interleukin-1beta, interleukin-6, transforming growth factor-beta and prostaglandin E(2) by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage 10(6):491–500. https://doi.org/10.1053/joca.2002.0528
Yan JY, Tian FM, Wang WY, Cheng Y, Song HP, Zhang YZ, Zhang L (2014) Parathyroid hormone (1–34) prevents cartilage degradation and preserves subchondral bone micro-architecture in guinea pigs with spontaneous osteoarthritis. Osteoarthritis Cartilage 22(11):1869–1877. https://doi.org/10.1016/j.joca.2014.07.013
Bellido M, Lugo L, Roman-Blas JA, Castaneda S, Calvo E, Largo R, Herrero-Beaumont G (2011) Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthritis Cartilage 19(10):1228–1236. https://doi.org/10.1016/j.joca.2011.07.003
Zhu S, Chen K, Lan Y, Zhang N, Jiang R, Hu J (2013) Alendronate protects against articular cartilage erosion by inhibiting subchondral bone loss in ovariectomized rats. Bone 53(2):340–349. https://doi.org/10.1016/j.bone.2012.12.044
Wakita T, Mogi M, Kurita K, Kuzushima M, Togari A (2006) Increase in RANKL: OPG ratio in synovia of patients with temporomandibular joint disorder. J Dent Res 85(7):627–632
Kaneyama K, Segami N, Sato J, Nishimura M, Yoshimura H (2003) Expression of osteoprotegerin in synovial tissue and degradation of articular cartilage: comparison with arthroscopic findings of temporomandibular joint disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 96(3):258–262. https://doi.org/10.1016/S1079210403003743
Kaneyama K, Segami N, Nishimura M, Sato J, Suzuki T, Fujimura K (2003) Osteoclastogenesis inhibitory factor/osteoprotegerin in synovial fluid from patients with temporomandibular disorders. Int J Oral Maxillofac Surg 32(4):404–407. https://doi.org/10.1054/ijom.2002.0363
Pilichou A, Papassotiriou I, Michalakakou K, Fessatou S, Fandridis E, Papachristou G, Terpos E (2008) High levels of synovial fluid osteoprotegerin (OPG) and increased serum ratio of receptor activator of nuclear factor-kappa B ligand (RANKL) to OPG correlate with disease severity in patients with primary knee osteoarthritis. Clin Biochem 41(9):746–749. https://doi.org/10.1016/j.clinbiochem.2008.02.011
Komuro H, Olee T, Kuhn K, Quach J, Brinson DC, Shikhman A, Valbracht J, Creighton-Achermann L, Lotz M (2001) The osteoprotegerin/receptor activator of nuclear factor kappaB/receptor activator of nuclear factor kappaB ligand system in cartilage. Arthritis Rheum 44(12):2768–2776
Kwan Tat S, Amiable N, Pelletier JP, Boileau C, Lajeunesse D, Duval N, Martel-Pelletier J (2009) Modulation of OPG, RANK and RANKL by human chondrocytes and their implication during osteoarthritis. Rheumatology 48(12):1482–1490. https://doi.org/10.1093/rheumatology/kep300
Moreno-Rubio J, Herrero-Beaumont G, Tardio L, Alvarez-Soria MA, Largo R (2010) Nonsteroidal antiinflammatory drugs and prostaglandin E(2) modulate the synthesis of osteoprotegerin and RANKL in the cartilage of patients with severe knee osteoarthritis. Arthritis Rheumat 62(2):478–488. https://doi.org/10.1002/art.27204
Hofbauer LC, Kuhne CA, Viereck V (2004) The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact 4(3):268–275
Edwards JR, Mundy GR (2011) Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol 7(4):235–243. https://doi.org/10.1038/nrrheum.2011.23
Shen P, Jiao Z, Zheng JS, Xu WF, Zhang SY, Qin A, Yang C (2015) Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Sci Rep 5:16244. https://doi.org/10.1038/srep16244
Shirakura M, Tanimoto K, Eguchi H, Miyauchi M, Nakamura H, Hiyama K, Tanimoto K, Tanaka E, Takata T, Tanne K (2010) Activation of the hypoxia-inducible factor-1 in overloaded temporomandibular joint, and induction of osteoclastogenesis. Biochem Biophys Res Commun 393(4):800–805. https://doi.org/10.1016/j.bbrc.2010.02.086
Jiao K, Niu LN, Li QH, Ren GT, Zhao CM, Liu YD, Tay FR, Wang MQ (2015) beta2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci Rep 5:12593. https://doi.org/10.1038/srep12593
Liu YD, Liao LF, Zhang HY, Lu L, Jiao K, Zhang M, Zhang J, He JJ, Wu YP, Chen D, Wang MQ (2014) Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthritis Cartilage 22(2):302–312. https://doi.org/10.1016/j.joca.2013.11.014
Zhang HY, Liu YD, Yang HX, Zhang M, Liao LF, Wan XH, Wang MQ (2015) Installing and thereafter removing an aberrant prosthesis elicited opposite remodelling responses in growing mouse temporomandibular joints. J Oral Rehabil 42(9):685–692. https://doi.org/10.1111/joor.12304
Embree M, Ono M, Kilts T, Walker D, Langguth J, Mao J, Bi Y, Barth JL, Young M (2011) Role of subchondral bone during early-stage experimental TMJ osteoarthritis. J Dent Res 90(11):1331–1338. https://doi.org/10.1177/0022034511421930
Ikeda Y, Yonemitsu I, Takei M, Shibata S, Ono T (2014) Mechanical loading leads to osteoarthritis-like changes in the hypofunctional temporomandibular joint in rats. Arch Oral Biol 59(12):1368–1376. https://doi.org/10.1016/j.archoralbio.2014.08.010
Yang T, Zhang J, Cao Y, Zhang M, Jing L, Jiao K, Yu S, Chang W, Chen D, Wang M (2015) Wnt5a/Ror2 mediates temporomandibular joint subchondral bone remodeling. J Dent Res 94(6):803–812. https://doi.org/10.1177/0022034515576051
Xu L, Guo H, Li C, Xu J, Fang W, Long X (2016) A time-dependent degeneration manner of condyle in rat CFA-induced inflamed TMJ. Am J Transl Res 8(2):556–567
Bolon B, Grisanti M, Villasenor K, Morony S, Feige U, Simonet WS (2015) Generalized Degenerative Joint Disease in Osteoprotegerin (Opg) Null Mutant Mice. Vet Pathol 52(5):873–882. https://doi.org/10.1177/0300985815586221
Shimizu S, Asou Y, Itoh S, Chung UI, Kawaguchi H, Shinomiya K, Muneta T (2007) Prevention of cartilage destruction with intraarticular osteoclastogenesis inhibitory factor/osteoprotegerin in a murine model of osteoarthritis. Arthritis Rheumat 56(10):3358–3365. https://doi.org/10.1002/art.22941
Jung J-K, Sohn W-J, Lee Y, Bae YC, Choi J-K, Kim J-Y (2014) Morphological and cellular examinations of experimentally induced malocclusion in mice mandibular condyle. Cell Tissue Res 355(2):355–363
Walker CG, Ito Y, Dangaria S, Luan X, Diekwisch TG (2008) RANKL, osteopontin, and osteoclast homeostasis in a hyperocclusion mouse model. Eur J Oral Sci 116(4):312–318. https://doi.org/10.1111/j.1600-0722.2008.00545.x
Glasson SS, Chambers MG, Van Den Berg WB, Little CB (2010) The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18(Suppl 3):S17–S23. https://doi.org/10.1016/j.joca.2010.05.025
Gosset M, Berenbaum F, Thirion S, Jacques C (2008) Primary culture and phenotyping of murine chondrocytes. Nat Protoc 3(8):1253–1260. https://doi.org/10.1038/nprot.2008.95
Blair-Levy JM, Watts CE, Fiorentino NM, Dimitriadis EK, Marini JC, Lipsky PE (2008) A type I collagen defect leads to rapidly progressive osteoarthritis in a mouse model. Arthritis Rheumat 58(4):1096–1106. https://doi.org/10.1002/art.23277
Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT (2006) Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 38(2):234–243. https://doi.org/10.1016/j.bone.2005.08.007
Patel V, Issever AS, Burghardt A, Laib A, Ries M, Majumdar S (2003) MicroCT evaluation of normal and osteoarthritic bone structure in human knee specimens. J Orthop Res 21(1):6–13. https://doi.org/10.1016/S0736-0266(02)00093-1
Chappard C, Peyrin F, Bonnassie A, Lemineur G, Brunet-Imbault B, Lespessailles E, Benhamou CL (2006) Subchondral bone micro-architectural alterations in osteoarthritis: a synchrotron micro-computed tomography study. Osteoarthritis Cartilage 14(3):215–223. https://doi.org/10.1016/j.joca.2005.09.008
Jiao K, Niu LN, Wang MQ, Dai J, Yu SB, Liu XD, Wang J (2011) Subchondral bone loss following orthodontically induced cartilage degradation in the mandibular condyles of rats. Bone 48(2):362–371. https://doi.org/10.1016/j.bone.2010.09.010
Bobinac D, Spanjol J, Zoricic S, Maric I (2003) Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans. Bone 32(3):284–290
Yamada K, Healey R, Amiel D, Lotz M, Coutts R (2002) Subchondral bone of the human knee joint in aging and osteoarthritis. Osteoarthritis Cartilage 10(5):360–369. https://doi.org/10.1053/joca.2002.0525
Knowles HJ, Moskovsky L, Thompson MS, Grunhen J, Cheng X, Kashima TG, Athanasou NA (2012) Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Arch 461(2):205–210. https://doi.org/10.1007/s00428-012-1274-3
McManus S, Chamoux E, Bisson M, Roux S (2012) Modulation of tumor necrosis factor related apoptosis-inducing ligand (TRAIL) receptors in a human osteoclast model in vitro. Apoptosis 17(2):121–131. https://doi.org/10.1007/s10495-011-0662-5
Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH (2012) Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PloS ONE 7(9):e45036. https://doi.org/10.1371/journal.pone.0045036
Romas E, Sims NA, Hards DK, Lindsay M, Quinn JW, Ryan PF, Dunstan CR, Martin TJ, Gillespie MT (2002) Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol 161(4):1419–1427. https://doi.org/10.1016/S0002-9440(10)64417-3
Acknowledgements
We wish to thank Haiming Du and Yunfei Wang for their assistance in animal handling. This study was funded by the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
DC conceived and designed the study, collected, assembled and interpreted the data, wrote the manuscript. YL collected the data, provided technical supports. ZL and PW provided technical supports. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
Danying Chen, Yi Liu, Zongxiang Liu, and Penglai Wang declare that they have no competing interests.
Human and Animal Rights and Informed Consent
Not applicable, as there were no human subjects. All animal protocols were approved by the Animal Use and Care Committee of Tongji University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, D., Liu, Y., Liu, Z. et al. OPG is Required for the Postnatal Maintenance of Condylar Cartilage. Calcif Tissue Int 104, 461–474 (2019). https://doi.org/10.1007/s00223-018-00510-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-00510-z