Abstract
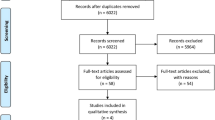
The aim of this study was to evaluate the morphological bone response in animal experiments by applying hydroxyapatite grafts in critical and non-critical size bone defects. Current report followed the guidelines established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Animal experiments were selected by assessing repair of bone defects with hydroxyapatite as bone graft and with blood clot only as control. Eight articles were identified in specialized literature and included in the meta-analysis. Statistical analysis was carried out with a random-effect model (p = 0.05). Subgroup analyses were further performed to investigate bone repair in critical and non-critical bone defects. Comprehensive analysis of bone repair outcome showed a statistically significant difference between hydroxyapatite and blood clot control (p < 0.05). Subgroup analyses showed statistically significant difference for critical bone defects (p < 0.05). No statistically significant difference was reported in non-critical bone defects (p > 0.05). Although animal studies revealed a high risk of bias and results should be interpreted with caution, the literature suggests that non-critical bone defects may heal spontaneously and without the need of a bone graft. Conversely, when critical-size defects are present, the use of hydroxyapatite bone graft improves the bone repair process.




Similar content being viewed by others
References
Gu Y, Wang G, Zhang X et al (2014) Biodegradable borosilicate bioactive glass scaffolds with a trabecular microstructure for bone repair. Mater Sci Eng C Mater Biol Appl 36:294–300. doi:10.1016/j.msec.2013.12.023
Chen FM, Liu X (2016) Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci 53:86–168. doi:10.1016/j.progpolymsci.2015.02.004
Gao C, Deng Y, Feng P et al (2014) Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci 15:4714–4732. doi:10.3390/ijms15034714
Tan L, Yu X, Wan P, Yang K (2013) Biodegradable materials for bone repairs: a review. J Mater Sci Technol 29:503–513. doi:10.1016/j.jmst.2013.03.002
Zhang J, Liu W, Schnitzler V et al (2014) Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater 10:1035–1049. doi:10.1016/j.actbio.2013.11.001
García-Gareta E, Coathup MJ, Blunn GW (2015) Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 81:112–121. doi:10.1016/j.bone.2015.07.007
Sheikh Z, Najeeb S, Khurshid Z et al (2015) Biodegradable materials for bone repair and tissue engineering applications. Materials 8:5744–5794. doi:10.3390/ma8095273
Ricciardi BF, Bostrom MP (2013) Bone graft substitutes: claims and credibility. Semin Arthroplast 24:119–123. doi:10.1053/j.sart.2013.07.002
Mahyudin F, Widhiyanto L, Hermawan H (2016) Biomaterials in orthopaedics. In: Advanced structured materials. pp 161–181
Zhang J, Liu W, Schnitzler V et al (2013) Review: calcium phosphate cements (CPCs) for bone substitution: chemistry, handling and mechanical properties. Acta Biomater 10:1035–1049. doi:10.1016/j.actbio.2013.11.001
Yu X, Tang X, Gohil SV, Laurencin CT (2015) Biomaterials for bone regenerative engineering. Adv Healthc Mater 4:1268–1285. doi:10.1002/adhm.201400760
Pepla E, Besharat LK, Palaia G et al (2014) Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol 5:108–114
Schortinghuis J, Ruben JL, Meijer HJA et al (2003) Microradiography to evaluate bone growth into a rat mandibular defect. Arch Oral Biol 48:155–160. doi:10.1016/S0003-9969(02)00172-3
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. In: Cochrane Collab. p Table 7.7.a: formulae for combining groups
Hooijmans CR, Rovers MM, de Vries RBM et al (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43. doi:10.1186/1471-2288-14-43
Ashby ER, Rudkin GH, Ishida K, Miller TA (1996) Evaluation of a novel osteogenic factor, bone cell stimulating substance, in a rabbit cranial defect model. Plast Reconstr Surg 98:420–426
Bilkay U, Alper M, Celik N et al (2004) Comparing the osteogenic capacities of bone substitutes: hydroxyapatite, high-density porous polyethylene, and bone collagen: a biochemical and histological analysis. J Craniofac Surg 15:585–593
Calasans-Maia MD, Ascoli FO, Novellino ATNA et al (2009) Avaliação histológica comparativa de reparo ósseo em tíbia de coelho tratada com xenoenxertos. Acta Ortop Bras 17:340–343. doi:10.1590/S1413-78522009000600005
De Girolamo L, Arrigoni E, Stanco D et al (2011) Role of autologous rabbit adipose-derived stem cells in the early phases of the repairing process of critical bone defects. J Orthop Res 29:100–108. doi:10.1002/jor.21184
Klinge B, Alberius P, Isaksson S, Jönsson J (1992) Osseous response to implanted natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J Oral Maxillofac Surg 50:241–249. doi:10.1016/0278-2391(92)90320-Y
Kucukkolbasi H, Mutlu N, Isik K et al (2009) Histological evaluation of the effects of bioglass, hydroxyapatite, or demineralized freeze-dried bone, grafted alone or as composites, on the healing of tibial defects in rabbits. Saudi Med J 30:329–333
Kühne JH, Bartl R, Frisch B et al (1994) Bone formation in coralline hydroxyapatite. Effects of pore size studied in rabbits. Acta Orthop Scand 65:246–252. doi:10.3109/17453679408995448
Lee SW, Kim SG, Balázsi C et al (2012) Comparative study of hydroxyapatite from eggshells and synthetic hydroxyapatite for bone regeneration. Oral Surg Oral Med Oral Pathol Oral Radiol 113:348–355. doi:10.1016/j.tripleo.2011.03.033
Lindholm TC, Gao TJ, Lindholm TS (1994) The role of autogeneic bone marrow in the repair of a skull trephine defect filled with hydroxyapatite granules in the rabbit. Int J Oral Maxillofac Surg 23:306–311. doi:10.1016/S0901-5027(05)80116-X
Abdul Razak NH, Al-Salihi KA, Samsudin AR (2004) An in vivo study of a locally-manufactured hydroxyapatite-based material as bone replacement material. Med J Malaysia 59(Suppl B):119–120
Sawada Y, Hokugo A, Yang Y et al (2011) A novel hydroxyapatite ceramic bone substitute transformed by ostrich cancellous bone: characterization and evaluations of bone regeneration activity. J Biomed Mater Res Part B Appl Biomater 98:217–222. doi:10.1002/jbm.b.31783
Turk AE, Ishida K, Jensen JA et al (1993) Enhanced healing of large cranial defects by an osteoinductive protein in rabbits. Plast Reconstr Surg 92:593–600
Zhou AJ-J, Clokie CML, Peel SAF (2013) Bone formation in algae-derived and synthetic calcium phosphates with or without poloxamer. J Craniofac Surg 24:354–359. doi:10.1097/SCS.0b013e318267ba3f
Andrade A, Sant’Ana D, Mendes Junior J et al (2013) Effects of cigarette smoke inhalation and coffee consumption on bone formation and osseous integration of hydroxyapatite implant. Braz J Biol 73:173–177. doi:10.1590/S1519-69842013000100018
Doll BA, Towle HJ, Hollinger JO et al (1990) The osteogenic potential of two composite graft systems using osteogenin. J Periodontol 61:745–750. doi:10.1902/jop.1990.61.12.745
Eftekhari H, Farahpour MR, Rabiee SM (2015) Histopathological evaluation of potential impact of beta-tricalcium phosphate (HA + beta-TCP) granules on healing of segmental femur bone defect. Bratisl Lek Listy 116:30–34
Hamerschmidt RR, dos Santos RF, Araújo JC et al (2011) Hydroxyapatite granules used in the obliteration of mastoid cavities in rats. Braz J Otorhinolaryngol 77:315–321
Moreira ASB, Pastoreli MT, Damasceno LHF, Defino HLA (2003) Estudo experimental da influência das dimensões dos grânulos de hidroxiapatita na integração óssea. Acta Ortopédica Bras 11:240–250. doi:10.1590/S1413-78522003000400007
Notodihardjo FZ, Kakudo N, Kushida S et al (2012) Bone regeneration with BMP-2 and hydroxyapatite in critical-size calvarial defects in rats. J Cranio-Maxillofacial Surg 40:287–291. doi:10.1016/j.jcms.2011.04.008
Park JW, Jang JH, Bae SR et al (2009) Bone formation with various bone graft substitutes in critical-sized rat calvarial defect. Clin Oral Implants Res 20:372–378. doi:10.1111/j.1600-0501.2008.01602.x
Rojbani H, Nyan M, Ohya K, Kasugai S (2011) Evaluation of the osteoconductivity of α-tricalcium phosphate, β-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J Biomed Mater Res Part A 98:488–498. doi:10.1002/jbm.a.33117
Sotto-Maior BS, Senna PM, Aarestrup BJV et al (2011) Effect of bovine hydroxyapatite on early stages of bone formation. Rev Odonto Ciência 26:198–292
Soccol AT, Bettega S, Noronha L et al (2006) Comparação entre os bioenxertos de hidroxiapatita de cálcio e submucosa de intestino delgado porcino no preenchimento de defeitos ósseos criados em mandíbula de ratos. Rev Bras Otorrinolaringol 72:195–199
Appleford MR, Oh S, Oh N, Ong JL (2009) In vivo study on hydroxyapatite scaffolds with trabecular architecture for bone repair. J Biomed Mater Res Part A 89:1019–1027. doi:10.1002/jbm.a.32049
Carvalho AL, Faria PEP, Grisi MFM et al (2007) Effect of granule size on the osteoconductivity of bovine and synthetic hydroxyapatite: a histologic and histometric study in dogs. J Oral Implantol 33:267–276. doi:10.1563/1548-1336(2007)33[267:EOGSOT]2.0.CO;2
Franco KL, Borges APB, Vilória MIV et al (2001) Hidroxiapatita sintética pura, hidroxiapatita sintética associada ao colágeno e hidroxiapatita sintética associada ao lipossoma como substitutos ósseos em defeitos provocados na tíbia de cães: aspectos da osteointegração à microscopia de luz transmitida. Arq Bras Med Vet e Zootec 53:431–436. doi:10.1590/S0102-09352001000400007
Lemperle SM, Calhoun CJ, Curran RW, Holmes RE (1998) Bony healing of large cranial and mandibular defects protected from soft-tissue interposition: a comparative study of spontaneous bone regeneration, osteoconduction, and cancellous autografting in dogs. Plast Reconstr Surg 101:660–672. doi:10.1097/00006534-199803000-00013
Buser D, Hoffmann B, Bernard JP, Lussi A, Mettler DSR (1998) Evaluation of filling materials in membrane protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin Oral Implant Res 9:137–150
Thorwarth M, Wehrhan F, Srour S et al (2007) Evaluation of substitutes for bone: comparison of microradiographic and histological assessments. Br J Oral Maxillofac Surg 45:41–47. doi:10.1016/j.bjoms.2006.03.013
Reedy BK, Pan FH, Kim WS et al (1999) Properties of coralline hydroxyapatite and expanded polytetrafluoroethylene membrane in the immature craniofacial skeleton. Plast Reconstr Surg 103:20–26. doi:10.1097/00006534-199901000-00005
Houshmand B, Rahimi H, Ghanavati F et al (2007) Boosting effect of bisphosphonates on osteoconductive materials: a histologic in vivo evaluation. J Periodontal Res 42:119–123. doi:10.1111/j.1600-0765.2006.00923.x
Nandi SK, Kundu B, Ghosh SK et al (2008) Efficacy of nano-hydroxyapatite prepared by an aqueous solution combustion technique in healing bone defects of goat. J Vet Sci 9:183–191. doi:10.4142/jvs.2008.9.2.183
Celik EU, Sirin TC, Ergucu Z et al (2008) Can different chlorhexidine agents be used as cavity disinfectants? Gen Dent 56:e33–e37
Schmitz JP, Hollinger JO (1986) The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. doi:10.1097/00003086-198604000-00036
LeGeros RZ (2008) Calcium phosphate-based osteoinductive materials. Chem Rev 108:4742–4753. doi:10.1021/cr800427g
Nandi SK, Kundu B, Mukherjee J et al (2015) Converted marine coral hydroxyapatite implants with growth factors: in vivo bone regeneration. Mater Sci Eng C Mater Biol Appl 49:816–823. doi:10.1016/j.msec.2015.01.078
Jun SH, Lee EJ, Jang TS et al (2013) Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. J Mater Sci Mater Med 24:773–782. doi:10.1007/s10856-012-4822-0
Xiao W, Fu H, Rahaman MN et al (2013) Hollow hydroxyapatite microspheres: a novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomater 9:8374–8383. doi:10.1016/j.actbio.2013.05.029
Hu J, Zhou Y, Huang L et al (2014) Effect of nano-hydroxyapatite coating on the osteoinductivity of porous biphasic calcium phosphate ceramics. BMC Musculoskelet Disord 15:114. doi:10.1186/1471-2474-15-114
Guo Y-P, Long T, Tang S et al (2014) Hydrothermal fabrication of magnetic mesoporous carbonated hydroxyapatite microspheres: biocompatibility, osteoinductivity, drug delivery property and bactericidal property. J Mater Chem B 2:2899–2909. doi:10.1039/c3tb21829e
Cooper GM, Mooney MP, Gosain AK et al (2010) Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast Reconstr Surg 125:1685–1692. doi:10.1097/PRS.0b013e3181cb63a3
Gosain AK, Song L, Yu P et al (2000) Osteogenesis in cranial defects: reassessment of the concept of critical size and the expression of TGF-beta isoforms. Plast Reconstr Surg 106:360–371. doi:10.1097/00006534-200008000-00018
Frame JW (1980) A convenient animal model for testing bone substitute materials. J Oral Surg 38:176–180
Hollinger J, Kleinschmidt J (1990) The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg 1:60–68. doi:10.1017/CBO9781107415324.004
Marsell R, Einhorn TA (2011) The biology of fracture healing. Injury 42:551–555. doi:10.1016/j.injury.2011.03.031
Loi F, Córdova LA, Pajarinen J et al (2016) Inflammation, fracture and bone repair. Bone 86:119–130. doi:10.1016/j.bone.2016.02.020
Roguska A, Hiromoto S, Yamamoto A et al (2011) Collagen immobilization on 316L stainless steel surface with cathodic deposition of calcium phosphate. Appl Surf Sci 257:5037–5045. doi:10.1016/j.apsusc.2011.01.018
Moura CCG, Souza MA, Dechichi P et al (2010) The effect of a nanothickness coating on rough titanium substrate in the osteogenic properties of human bone cells. J Biomed Mater Res, Part A 94A:103–111. doi:10.1002/jbm.a.32661
Lee Y-J, Ko JS, Kim H-M (2006) The role of cell signaling defects on the proliferation of osteoblasts on the calcium phosphate apatite thin film. Biomaterials 27:3738–3744. doi:10.1016/j.biomaterials.2006.02.032
Mills LA, Simpson AHRW (2012) In vivo models of bone repair. J Bone Joint Surg Br 94:865–874. doi:10.1302/0301-620X.94B7.27370
von Rechenberg B (2014) Animal models in bone repair. Drug Discov Today Dis Model 13:23–27. doi:10.1016/j.ddmod.2015.02.001
Kilkenny C, Browne WJ, Cuthill IC et al (2013) Improving bioscience research reporting: the arrive guidelines for reporting animal research. Animals 4:35–44. doi:10.3390/ani4010035
Acknowledgements
CECS wants to thank PRODEP/México for the scholarship.
Author information
Authors and Affiliations
Contributions
HLO, WLOR and EP designed the study. HLO and CECS prepared the first draft of the paper. NLVC, AFS, TNG and OAD contributed to the experimental work. WLOR was responsible for statistical analysis of the data. HLO, CECS, WLOR and EP prepared the final draft of the manuscript. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Funding
This study was financed by Coordination for the Improvement of Higher Education Personnel, CAPES/MEC-Brazil.
Corresponding author
Ethics declarations
Conflict of interest
Authors Héllen L. Oliveira, Wellington L. O. Rosa, Carlos E. Cuevas-Suárez, Neftali L. V. Carreño, Adriana F. Silva, Thomas N. Guim, Odir A. Dellagostin and Evandro Piva declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Oliveira, H.L., Da Rosa, W.L.O., Cuevas-Suárez, C.E. et al. Histological Evaluation of Bone Repair with Hydroxyapatite: A Systematic Review. Calcif Tissue Int 101, 341–354 (2017). https://doi.org/10.1007/s00223-017-0294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0294-z