Abstract
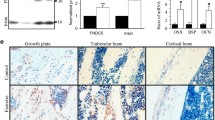
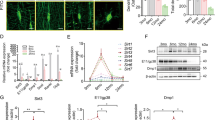
Intermittent parathyroid hormone (iPTH) treatment and mechanical loading are osteoanabolic stimuli that are partially mediated through actions on G protein-coupled receptors (GPCRs). GPCR signaling can be altered by heterotrimeric G protein Gα subunits levels, which can therefore lead to altered responses to such stimuli. Previous studies have suggested that enhanced signaling through the Gαq/11 pathway inhibits the osteoanabolic actions of PTH. The influence of Gαq/11 signaling on mechanotransduction, however, has not been reported in vivo. Using transgenic mice that specifically overexpress Gα11 in osteoblast lineage cells (G11-Tg mice), we investigated the skeletal effects of elevated Gα11 levels on iPTH and mechanical loading by treadmill exercise. Both regimens increased trabecular and cortical bone in Wild-Type (WT) mice as a result of increased bone formation. In G11-Tg mice, there was no change in trabecular or cortical bone and no increase in bone formation in response to iPTH or exercise. While exercise reduced osteoclast parameters in WT mice, these changes were diminished in G11-Tg mice as expression of M-csf and Trap remained increased. Collectively, our results suggest that osteoblastic upregulation of Gα11 is inhibitory to osteoanabolic actions of both PTH and exercise, and that its suppression may be a promising target for treating bone loss.




Similar content being viewed by others
References
Bowler WB, Gallagher JA, Bilbe G (1998) G-protein coupled receptors in bone. Front Biosci 3:769–780
Weinstein LS, Chen M, Xie T, Liu J (2006) Genetic diseases associated with heterotrimeric G proteins. Trends Pharmacol Sci 27:260–266
Datta NS, Abou-Samra AB (2009) PTH and PTHrP signaling in osteoblasts. Cell Signal 21:1245–1254
Hodsman AB, Bauer DC, Dempster DW et al (2011) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26(5):688–703
Howe TE, Shea B, Dawson LJ et al (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev Issue 7:CD000333
Warner SE, Shea JE, Miller SC, Shaw JM (2006) Adaptations in cortical and trabecular bone in response to mechanical loading with and without weight bearing. Calcif Tissue Int 79:395–403
Alvarenga EC, Rodrigues R, Caricati-Neto A et al (2010) Low-intensity pulsed ultrasound-dependent osteoblast proliferation occurs by via activation of the P2Y receptor: role of the P2Y1 receptor. Bone 46:355–362
Katz S, Boland R, Santillán G (2006) Modulation of ERK 1/2 and p38 MAPK signaling pathways by ATP in osteoblasts: involvement of mechanical stress-activated calcium influx, PKC and Src activation. Int J Biochem Cell Biol 38:2082–2091
Gardinier JD, Mohamed F, Kohn DH (2014) PTH signaling during exercise contributes to bone adaptation. J Bone Miner Res 30(6):1053–1063
Ishizuya T, Yokose S, Hori M et al (1997) Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest 99(12):2961–2970
Li X, Liu H, Qin L et al (2007) Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem 282:33086–33097
Ogata N, Shinoda Y, Wettschureck N et al (2011) G alpha(q) signal in osteoblasts is inhibitory to the osteoanabolic action of parathyroid hormone. J Biol Chem 286:13733–13740
Cruz Dela A, Mattocks M, Sugamori KS et al (2014) Reduced trabecular bone mass and strength in mice overexpressing Gα11 protein in cells of the osteoblast lineage. Bone 59:211–222
Cruz Dela A, Grynpas MD, Mitchell J (2016) Elevated Gα11 expression in osteoblast lineage cells promotes osteoclastogenesis and leads to enhanced trabecular bone accrual in response to pamidronate. Am J Physiol Endocrinol Metab 310(10):E811–E820
Dempster DW, Compston JE, Drezner MK et al (2012) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17
Pierroz DD, Bonnet N, Baldock PA et al (2010) Are osteoclasts needed for the bone anabolic response to parathyroid hormone? A study of intermittent parathyroid hormone with denosumab or alendronate in knock-in mice expressing humanized RANKL. J Biol Chem 285:28164–28173
Mori T, Okimoto N, Sakai A et al (2003) Climbing exercise increases bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J Bone Miner Res 18:2002–2009
Iura A, McNerny EG, Zhang Y et al (2015) Mechanical loading synergistically increases trabecular bone volume and improves mechanical properties in the mouse when BMP signaling is specifically ablated in osteoblasts. PLoS ONE 10(10):e0141345
McAteer ME, Niziolek PJ, Ellis SN et al (2010) Mechanical stimulation and intermittent parathyroid hormone treatment induce disproportional osteogenic, geometric, and biomechanical effects in growing mouse bone. Calcif Tissue Int 86:389–396
Rubin J, Fan X, Biskobing DM et al (1999) Osteoclastogenesis is repressed by mechanical strain in an in vitro model. J Orthop Res 17:639–645
Kim DW, Lee HJ, Karmin JA et al (2006) Mechanical loading differentially regulates membrane-bound and soluble RANKL availability in MC3T3-E1 cells. Ann N Y Acad Sci 1068:568–572
Thompson WR, Rubin CT, Rubin J (2012) Mechanical regulation of signaling pathways in bone. Gene 503:179–193
Rubin J, Murphy TC, Rahnert J et al (2006) Mechanical inhibition of RANKL expression is regulated by H-Ras-GTPase. J Biol Chem 281:1412–1418
Rubin J, Murphy TC, Zhu L et al (2003) Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J Biol Chem 278:34018–34025
Saunders MM, Taylor AF, Du C et al (2006) Mechanical stimulation effects on functional end effectors in osteoblastic MG-63 cells. J Biomech 39(8):1419–1427
Suzuki K, Nakaji S, Yamada M et al (2002) Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev 8:6–48
Compton JT, Lee FY (2014) A review of osteocyte function and the emerging importance of sclerostin. J Bone Joint Surg Am 96:1659–1668
Kramer I, Loots GG, Studer A et al (2010) Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25:178–189
Leupin O, Kramer I, Collette NM et al (2007) Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 22:1957–1967
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37:148–158
Spatz JM, Wein MN, Gooi JH et al (2015) The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J Biol Chem 290:16744–16758
Baek Y-S, Haas S, Hackstein H et al (2009) Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol 10:18
Tu X, Rhee Y, Condon KW et al (2012) Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50:209–217
Romanello M, Pani B, Bicego M, D’Andrea P (2001) Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun 289:1275–1281
Orriss IR, Utting JC, Brandao-Burch A et al (2007) Extracellular nucleotides block bone mineralization in vitro: evidence for dual inhibitory mechanisms involving both P2Y2 receptors and pyrophosphate. Endocrinology 148:4208–4216
Orriss IR, Key ML, Hajjawi MOR, Arnett TR (2013) Extracellular ATP released by osteoblasts is a key local inhibitor of bone mineralisation. PLoS ONE 8:e69057
Regard JB, Cherman N, Palmer D et al (2011) Wnt/β-catenin signaling is differentially regulated by Gα proteins and contributes to fibrous dysplasia. Proc Natl Acad Sci USA 108:20101–20106
Li G, Iyengar R (2002) Calpain as an effector of the Gq signaling pathway for inhibition of Wnt/beta -catenin-regulated cell proliferation. Proc Natl Acad Sci USA 99:13254–13259
Acknowledgments
This study was supported by a grant to JM from the Canadian Institutes of Health Research CIHR MOP-115064. ADC was supported by scholarships from the Toronto Centre for Musculoskeletal Research and the Ontario Graduate Scholarship fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ariana Dela Cruz, Marc D. Grynpas and Jane Mitchell declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All applicable international, national and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Dela Cruz, A., Grynpas, M.D. & Mitchell, J. Overexpression of Gα11 in Osteoblast Lineage Cells Suppresses the Osteoanabolic Response to Intermittent PTH and Exercise. Calcif Tissue Int 99, 423–434 (2016). https://doi.org/10.1007/s00223-016-0158-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0158-y